Let there be light! (History of Optogenetics)
Hi guys! Today I would like to give you broader perspective about optogenetics – novel scientific method used to control activity of cells in space and time. I already created brief introduction on this matter, you can read it HERE. Now I would like to be more elaborate and explain this technique in higher detail.
So sit down and enjoy the story.
In 1979 Francis Crick, Nobel prize winner and co-discoverer of double helical structure of DNA, proposed the need for a tool in neurobiological research that could selectively activate only one particular type of neurons and leave other neurons intact. Even though electric stimulation allows high temporal precision, it lacks the ability to stimulate only one specific cell. On the other hand, chemical agents do have the ability of temporal precision, but they are too slow to be effectively used to control dynamic cells like neurons in time. Optogenetics combines the benefits from both electrical and chemical stimulation – the precise spatio-temporal control of cells. Crick himself predicted the usage of light in neurobiology.
When Crick delivered his thoughts about the necessity of new, better tools for neural control, several molecules responsive to light had already been discovered. In 1971 scientists discovered a rhodopsin-like transmembrane protein in Halobacterium halobium that responded by the change of absorbance after light stimulation. It was named bacteriorhodopsin due to its similarity to rhodopsin in the retina of vertebrates. Later another type of opsin- halorhodopsin – was discovered. These microbial opsins function as ion pumps or channels and therefore have the ability to change the membrane potential of a cell- the same principle is used by neurons to deliver signals.
Even though several tools that could solve the need for better neurobiological tools had already been discovered at the time when Crick proposed this need, it was mainly due to the great gap between the study of the vertebrae brain and microbial proteins that the discovery of method such as optogenetics slowed down. The first experiments that resembled the current optogenetics used opsins from higher animals such as mammals. Even though microbial and vertebrae opsins share structural homology, the mechanism of their function is different. Because of this, the effective usage of neurons that expressed vertebrae opsins was temporally insufficient. In 2002 other microbial opsins- channelrhodopsin and channelrhodopsin-2 – were discovered.
A scientific breakthrough in 2005 changed everything. Channelrhodopsin-2 (ChR2) was expressed in neurons for the first time. Stimulation with blue light caused depolarization of these neurons. This technique was non-invasive and could be controlled in space and time. This was the corner stone for optogenetics (the method was titled optogenetics in 2006). In the last decade there has been a massive amount of new research conducted. The most prominent includes the usage of an optical fibre neural-interface that could import light into the skull of rodents in vivo, the usage of halorhodpsins to silence neuronal activity, or the modification of intracellular signalling via optogenetics.
The Nature termed optogenetics as method of the year 2010. In 2013 The Brain Prize was given to six scientists that discovered or mostly widened the usage of optogenetics - Ernst Bamberg, Ed Boyden, Karl Deisseroth, Peter Hegemann, Gero Miesenböck and Georg Nagel.
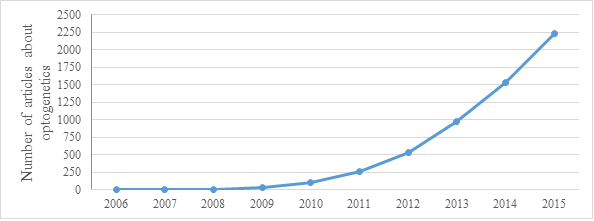
As you can see on this graph from 2015 the number of articles about optogenetics grows rapidly.
That is all from me for today, hopefully you did enjoy reading my article. Next time I will take closer look on components of optogenetic research as well as light responsive proteins.
References:
https://en.wikipedia.org/wiki/Optogenetics
https://www.ncbi.nlm.nih.gov/pubmed/16116447
https://www.sciencedirect.com/science/article/pii/S0896627311005046
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3155186/
https://web.stanford.edu/group/dlab/media/papers/deisserothNatNeurosciCommentary2015.pdf
https://www.ncbi.nlm.nih.gov/pubmed/24054067

