The Periodic Table of Mendeleev and Moseley
A reddish stain on a piece of cloth... a bit of dust accumulated in the wrinkles of a man's clothes... mud traces in the corner of a room... a bit of pipe tobacco in the scene of the crime...
What will these details mean?
For the detective Sherlock Holmes (creation of the British author Arthur Conan Doyle), they were clues to the most mysterious crimes imaginable.
Holmes could solve many amazing crimes by means of evidence. The success of Holmes had a simple and solid base: logical thinking combining with knowledge of chemistry.
With this knowledge he classified and analyzed key substances for the mysteries. Holmes was a master in the use of scientific principles to solve crimes.
Somehow, your daily life is similar to a good detective novel. Unanswered questions and unexplained problems challenge you all the time.
Chemists are also good detectives. Collect evidence, analyze data, follow clues and make predictions. That happened with the development of the periodic table of the elements.
 Dmitri Mendeleev imag source
Dmitri Mendeleev imag sourceThe detective of this fascinating story was the Russian chemist Dmitri Mendeleev. He discovered a huge collection of data on the 63 elements known in the mid-nineteenth century. His clues were the physical and chemical properties of those elements.
Mendeleev suspected that there was some order or relationship between all the elements. He was convinced that he could group them according to their properties.
In his search, Mendeleev first organized his data. For each known element he made a card in which he wrote the properties of the element: atomic mass, density, color and melting point. I also include the valence or link strength of the element.
Always looking for a pattern, Mendeleev arranged the cards in increasing order of atomic mass. If I started with lithium, the next would be beryllium. Then, boron, carbon, nitrogen, oxygen and fluorine would come.
With the cards arranged in this order, he noticed the extraordinary pattern of the valences: 1 2 3 4 3 2 1. Every seven elements, the pattern was repeated.
He also saw something even more amazing: by arranging them like this, the elements remained in columns, one under the other. All the elements of a column had the same valence, and to a greater surprise they all showed similar physical and chemical properties.
Mendeleev said:
"The properties of the elements are periodic functions of their atomic masses"
Mendeleev designed a periodic table with the elements arranged in increasing order of atomic mass. Confident in the accuracy of his discovery, he left spaces in the table so that the known elements had a place in the appropriate column.
Then he risked predicting that the empty places would be completed by new elements to be discovered, and he also predicted the physical and chemical properties of the unknown elements. And if that was not enough, he was right.
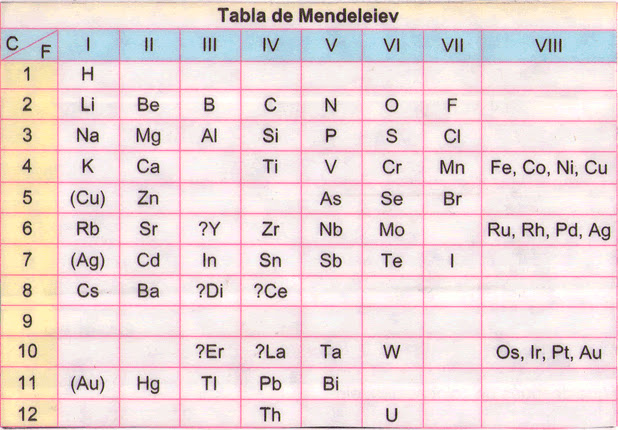
Despite the importance of Mendeleev's work, his periodic table was not perfect. By putting the elements in order of increasing atomic mass, several seemed out of place in terms of their properties.
 Henry Moseley imag source
Henry Moseley imag sourceThe answer to the problem did not become evident until 50 years after Mendeleev had developed his periodic table.
Henry Moseley determined for the first time the atomic numbers of the elements. As you remember, the atomic number of an element is the number of protons in the nucleus of each atom of that element.
The discovery of atomic numbers led to an important change in Mendeleev's periodic atabla. It was found that when the elements are arranged in ascending order of atomic number, the elements that have similar physical and chemical properties are in their correct place without exception.
Mendeleev's periodic table was replaced by the modern periodic table. The periodic law is the basis of the modern periodic table.
The periodic law states that the physical and chemical properties of the elements are periodic functions of their atomic numbers.
The periodic table of the elements is one of the most important instruments that scientists have, especially chemists. Why?
Because the periodic table is a classification system to organize large amounts of information in a useful and meaningful way.
The periodic table organizes the elements in a particular way. Much of the information about an element can be deduced by its position in the periodic table. Also, you can predict which elements another will react chemically with.
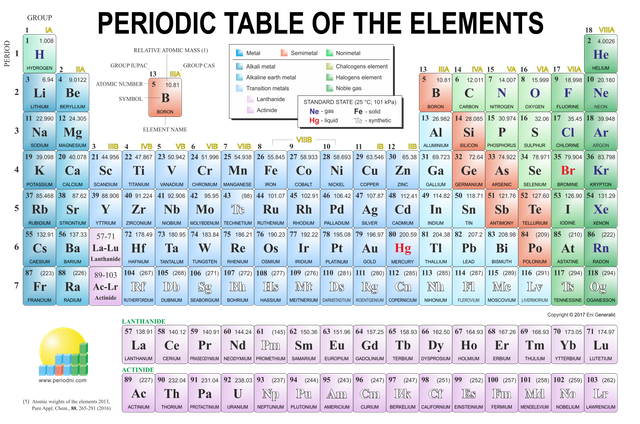
The periodic table consists of vertical columns of elements. Each column is numbered. There are eighteen main columns of elements called groups or families. The elements within the same group or family have similar but not identical properties.
The horizontal rows of the elements are called period. Unlike the elements of a family, the elements of a period do not have similar properties. The chemical symbol of an element consists of one or two letters. If it is a single letter, it is always capitalized; if there are two, the first is always capitalized and the second is lower case.
As you can see in the table above, there are seven element periods. You can also see that a row has been separated from Period 6 and a row from 7. Although these two rows are shown below the main part of the table, they are part of it. They have been separated so that the table is shorter and easier to read. These two rows are elements of Rare Earth.

Each square of the periodic table contains important information about an element:
atomic number, chemical symbol, name and atomic mass.
As you well know, there are several types of elements. But of the 109 known elements, 88 are metallic or metal-like elements.

Metals
Metals can be easily identified by their physical properties. Since most metals allow heat and electricity to move easily through them, they are good conductors of heat and electricity. gif source
No metals
In general, the physical and chemical properties of non-metals tend to be opposite to those of metals. They do not conduct heat or electricity; They are fragile and break easily. They can not be converted into wires or thin sheets. Non-metals as a whole are not as easy to recognize as metals.
The metalloids
Also called semimetal, are the elements that have properties of metals and nonmetals. All metalloids are solids that can shine or be opaque. They conduct heat and electricity better than non-metals, but not as well as metals.
So I finish my walk through the Periodic Table that gave us many headaches at school...
While looking for images of the periodic table I got myself with this material...

Credits: elements.wlonk.com
I think it can be very useful to teach children in schools, because it is easier to understand through images. Here you can see it in high definition.
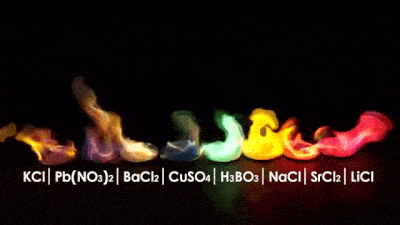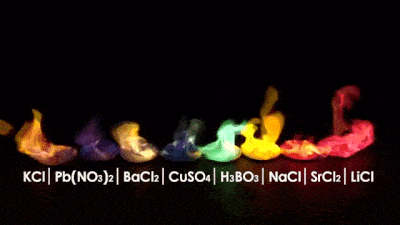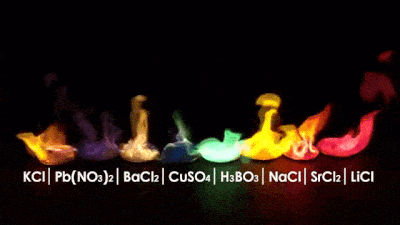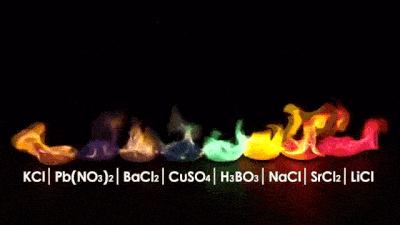
Sources:
1. Henry Moseley - Wikipedia 2. Generalidades de la Tabla Periodica 3. La Tabla Periódica Historia 4. Tabla Periódica - Wikipedia

Being A SteemStem Member
Very nic post.
Meendeleevs idea has really helped simplify the elements to students, nice work here thanks for sharing
Thanks to him we could know elements, some people is really important for human kind.
I agree with you can be better if we teach this with HD images to kids.
Very important information(;
Helpful article, thank you.

Another way to remember some elements!!!!
Among the very many illustrations of the famous table of elements I would recommand the amazing book (historical stories type) of PRIMO LEVI: "Il sistema periodico " (1975) established as the "best science book ever" by the Royal Institution of England in 2006.
Systematic and instructive text, nicely adapted for pupils' learning. Thank you for sharing!
Excellent!
excellente