Overview of Ozone layer Depletion
Hello all ,
This time i'm back to discuss about effects in atmosphere.I can't discuss about all effects but i'm here to describe you all about the ozone layer depletion ,which is one of the major among all effects.
About ozone:-
Ozone is odorless,colourless gas composed of three atoms of oxygen at an certain height.Ozone layer is naturally formed by short wavelength ultraviolet radiations in the upper stratosphere at an height of 35 km-50 km.
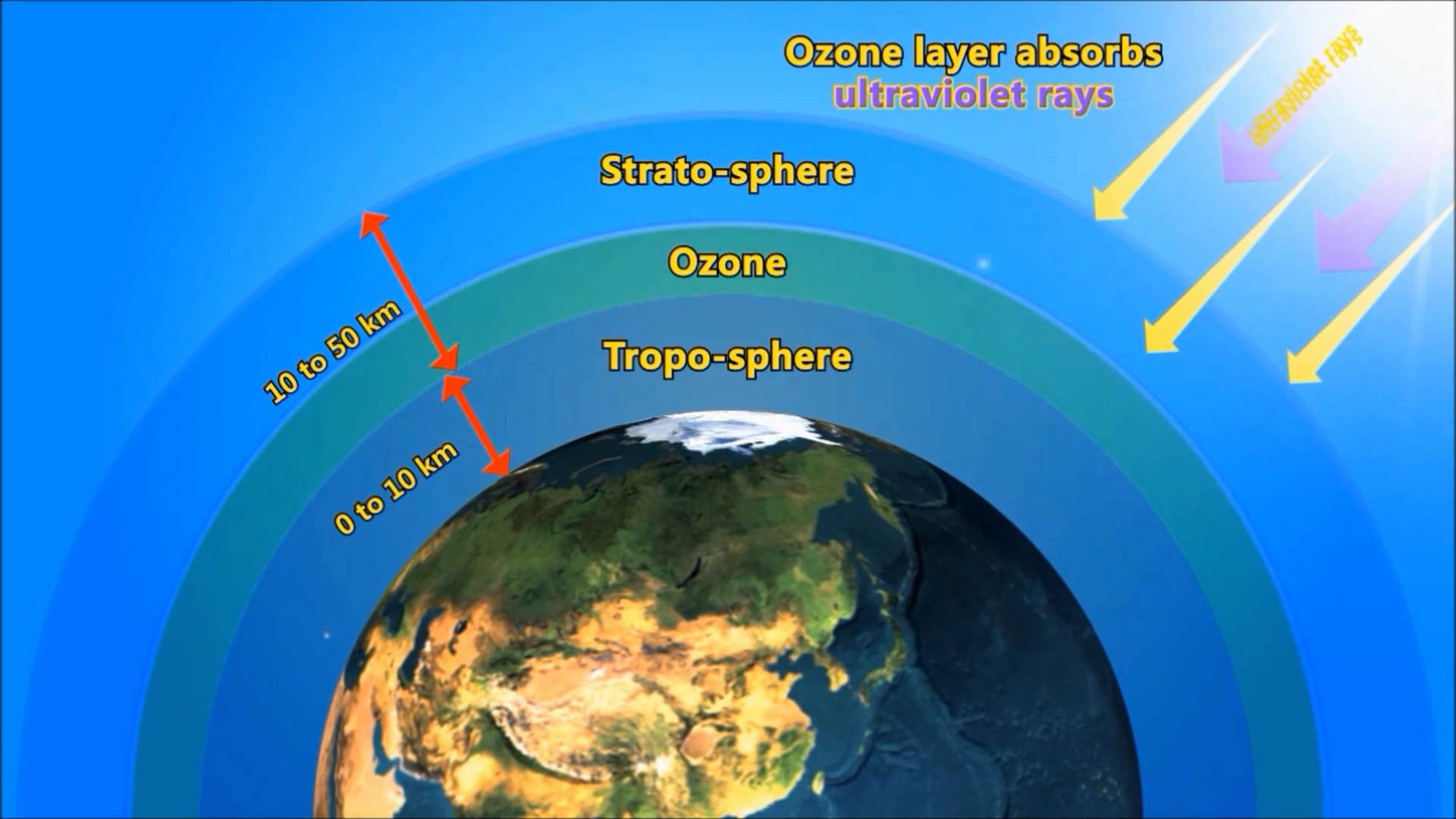
wavelength lessthan 240nm are absorbed by normal oxygen molecules which dissociates to give O atoms.The O atoms in combination with other oxygen moleculesproduce oxygen.
ozone layer prevents the harmful UV-rays from entering into earth's surface.
Ozone layer is not uniform throughout the earth.It is with different thickness at different areas.Till now,Antarctica has the biggest ozone hole followed by the Arctic region and the Tibetan plateau
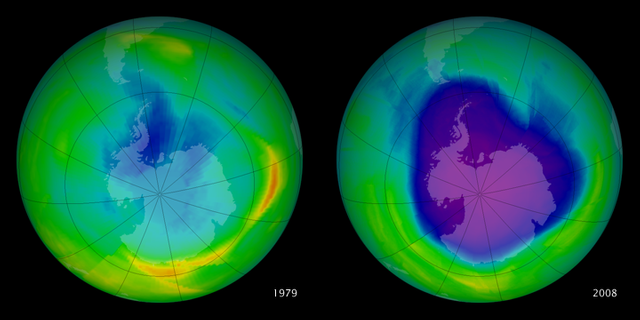
The blue region indicates the ozone hole formed in the specified area.Above picture gives us information about the increase in ozone layer at specified area in an certain period.
Factors Reasonable:-
Major components reasonable for depletion of ozone are Chlorofloro carbons or CFC's and Hydrofloro carbons or HFC's.these substances are Non-toxic,Non-flammable,Non-reactive with other chemical compounds.These chemicals consists of Chlorine or Bromine atoms which are reactive when they are in free state.
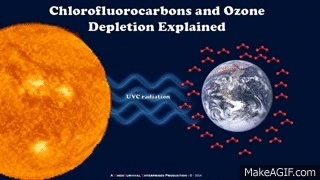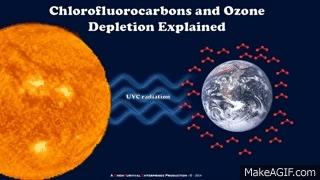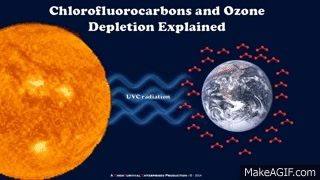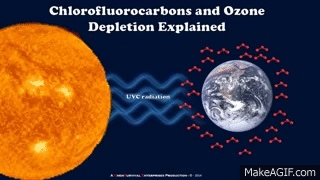
GIF source
Sources:-
For,
- CFC: Refrigerants,propellant in a aerosol spray.
- HFC: Refrigerants,blowing agents.
- BFC: Fire extinguisher.
Rather than CFC and HFC ozone layer is also affected by Nitrogen oxides such as Nitrous oxide which is very reactive with ozone. Since 1975,the ozone hole has increased in size due to depletion the layer.
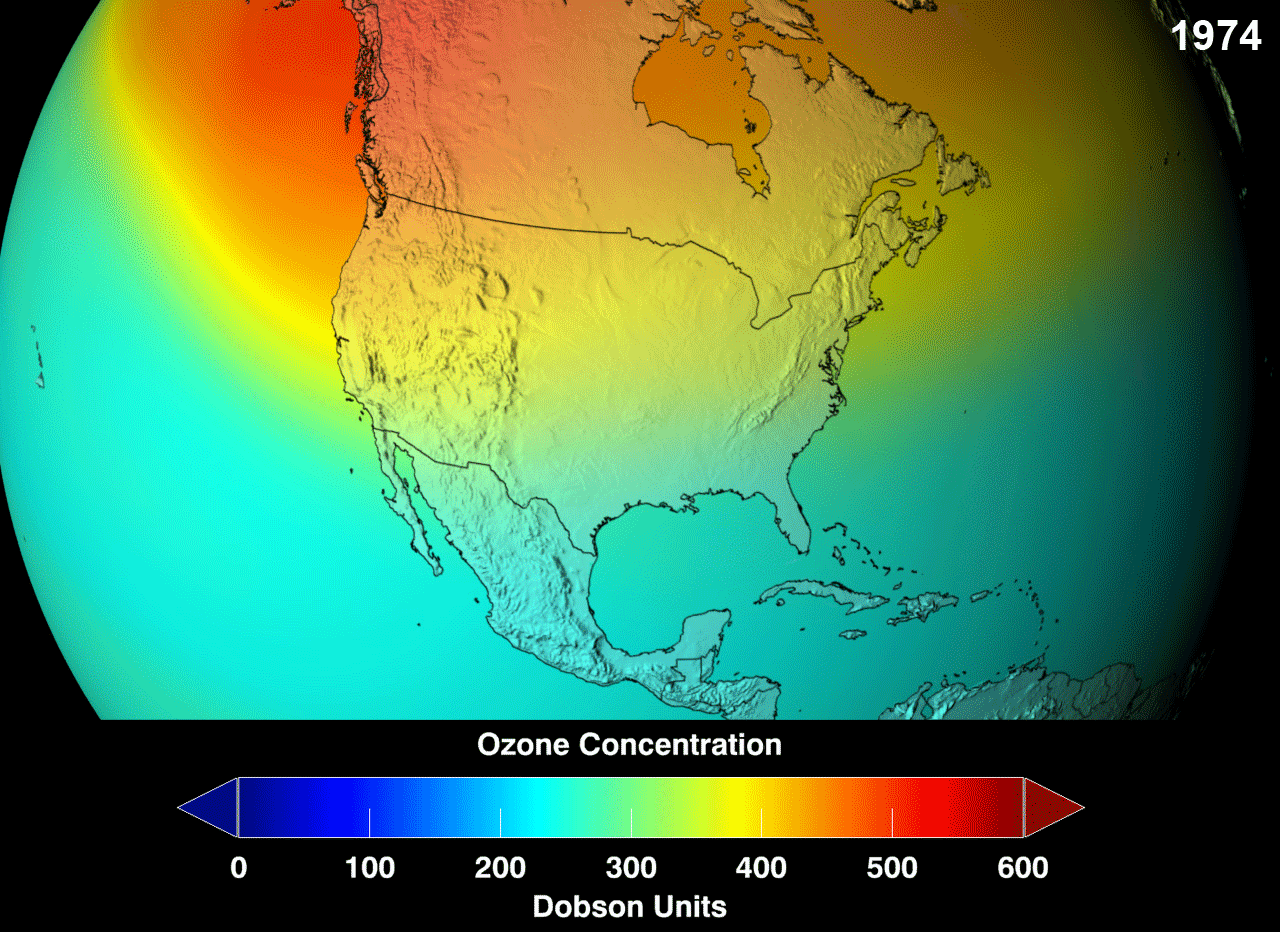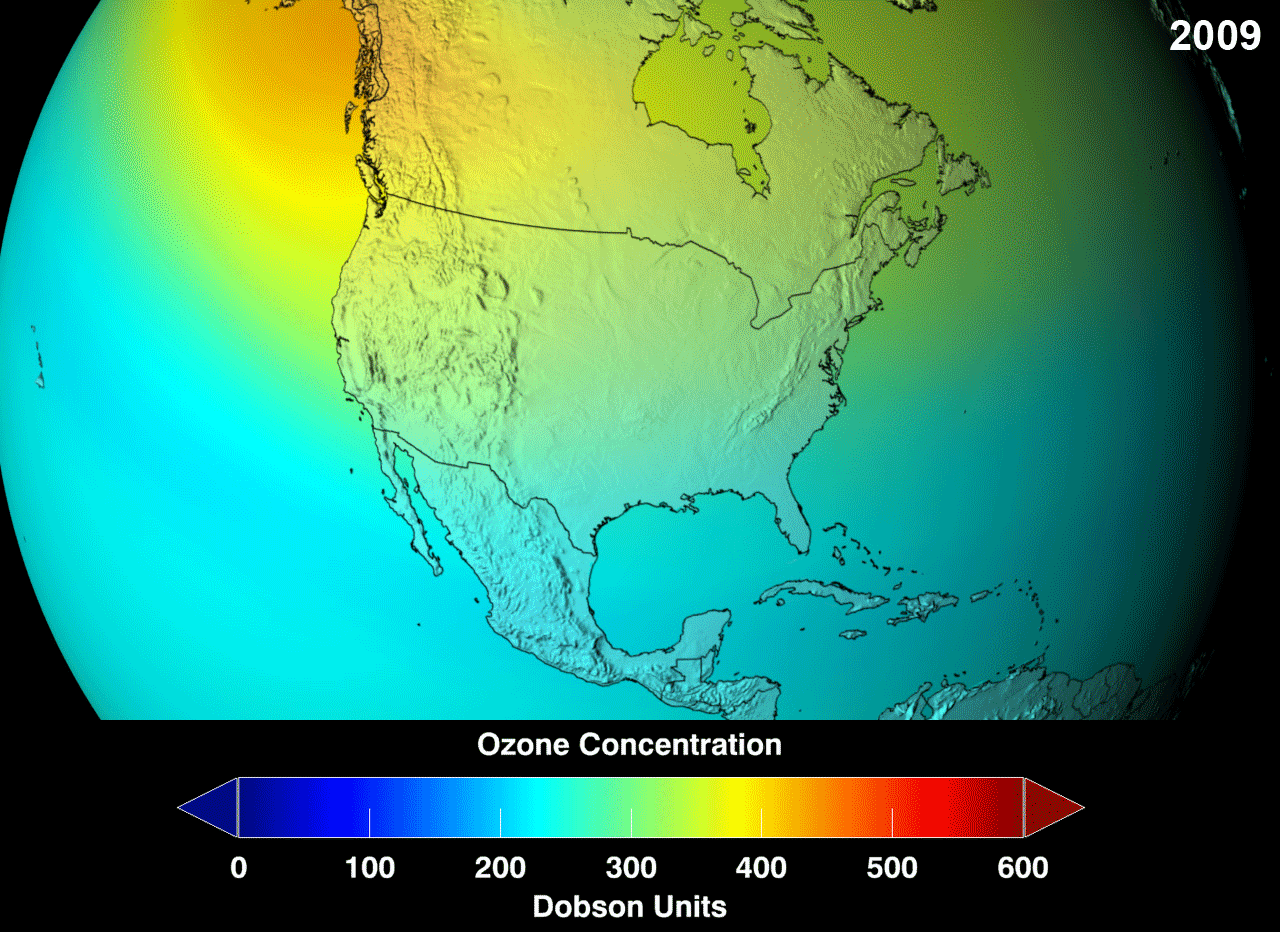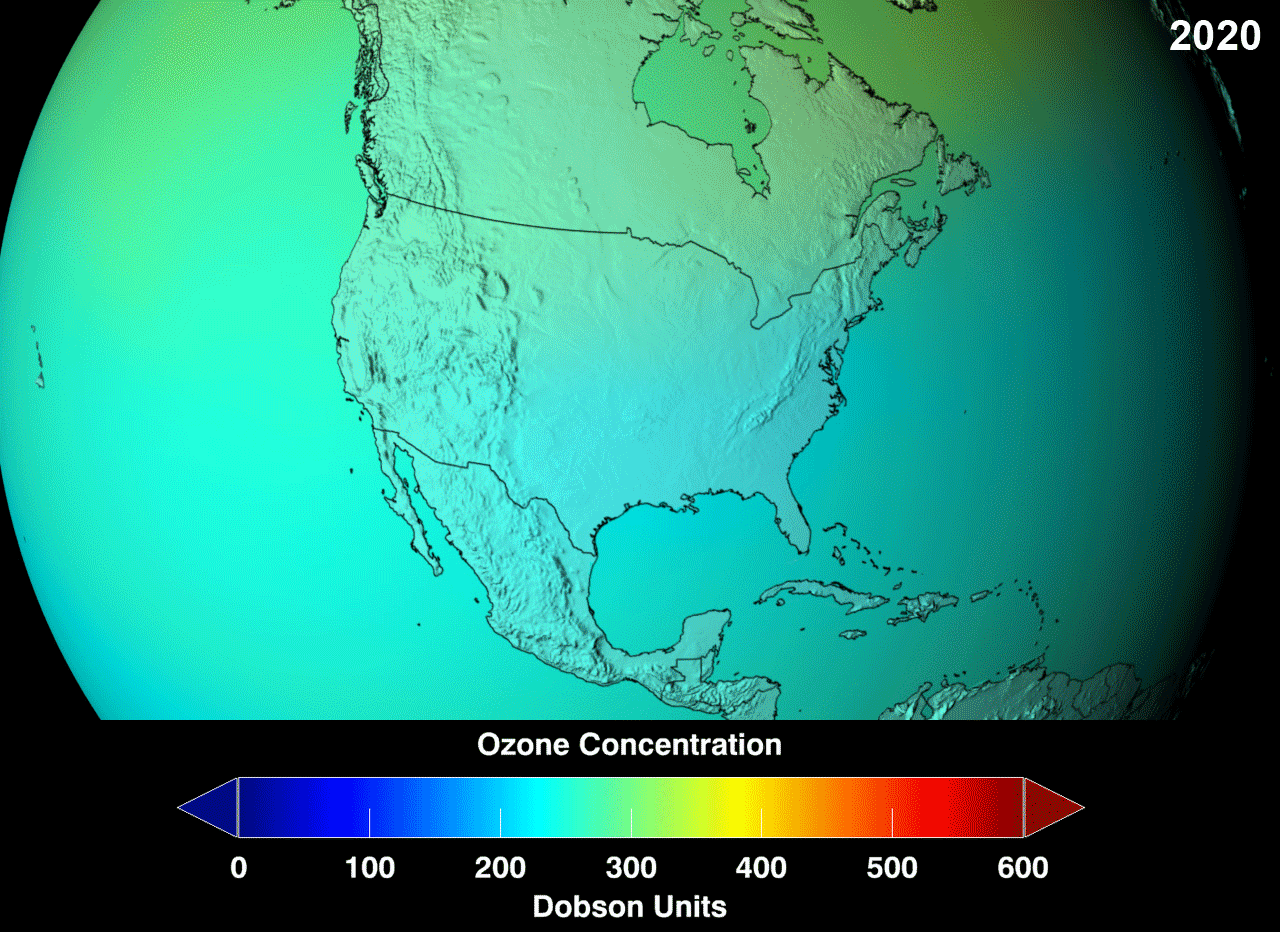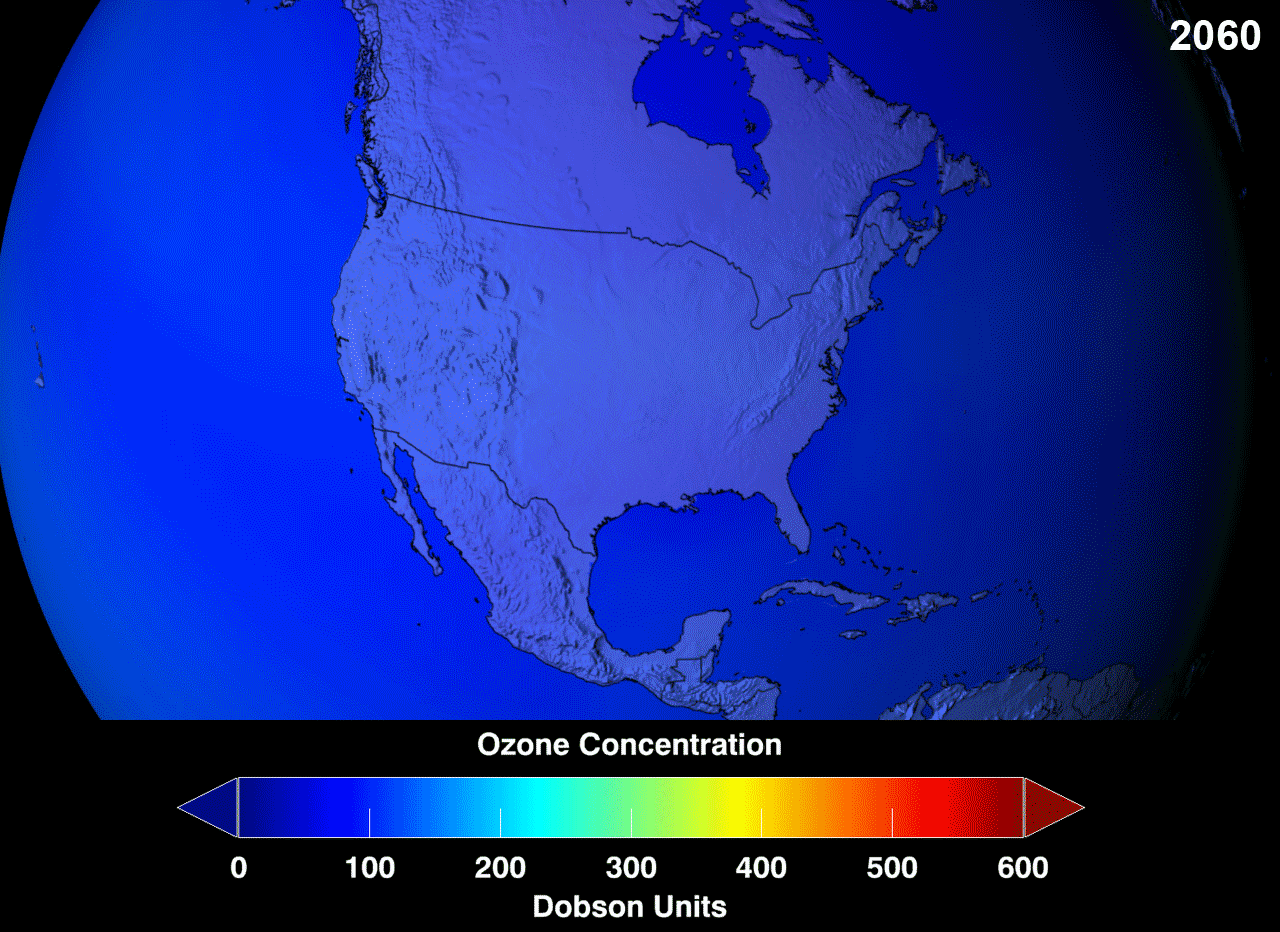
Ozone layer Depletion Process :-
The balance between the creation and removal of ozone is affected by increasing concentration of chlorine,nitrogen ,bromine,hydroxides etc...
Ozone is a highly unstable molecule that readily donates its extra oxygen molecule to free radical elements such as nitrogen, hydrogen,bromine and chlorine.
Ozone depletion by CFC,
CI + O3 -> CIO + O2 CIO + O -> Cl + O2
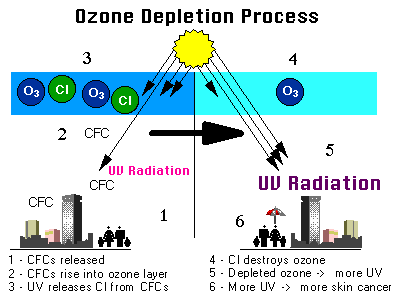
Each atomof chlorine can attack several ozone molecules.
Ozone depletion by NO,
O3 + NO -> O2 + NO2 O3 + NO2 -> O2 + NO3
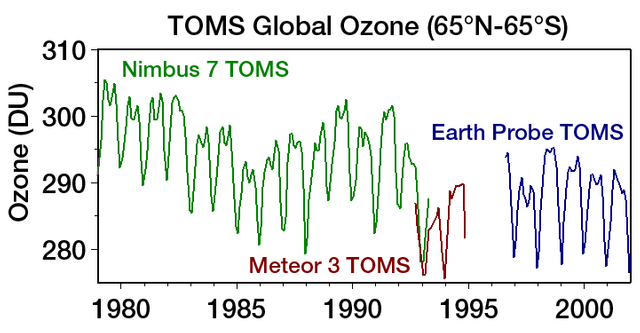
Ozone hole is generally expressed in the terms called Dosbon units which is represented as DU.The presence of Ozone in the atmosphere can be measured by the instrument Total Ozone Mapping Spectrometer (TOMS).
Advancements :-
BY 1985,ozone is got reduced upto 70% and it is continuing till date.A 2005 IPPC preview gave an review about the Ozone calculations and presence was approximately stabilized.In 2017,NASA proudly announces that there will be no Ozone hole by 2070.
Consequences :-
There are more effects due to the ozone depletion in the atmosphere .
Human health :
- Now a days,it is estimated there is an rapid increase in the skin cancer due to decrease in ozone layer.Among them,the most common type is Non-melanoma which is very effective to human body.
- Other than skin cancer there is possible of another skin disease known as reddening of skin in sunshine.
- Rather than skin diseases there will be an another major effect i.e Eye disorders like cataract and blindness.
- A person's defence against depends on his immune system.If a person get affected to UV radiation ,he will reduces the effectivness of immune system.
Effect on climate:-
- Depletion of Ozone layer leads to increase in the absorption of UV rays by the earth surface.This increases the temperature of earth's surface.
- It leads to increase in hydrogen peroxide in the troposphere which leads to the acid rain.
Terrestrial Ecosystems:-
- UV rays are harmful to other forms of wildlife particularly small plants and animals living in the sea called Plankton.
- UV rays damages certain crops like Rice, Soyabeans,Cotton,Wheat etc..
- These can damage polymers used in paint, clothing and other materials.
Control measures :-
- Replacing CFC's by materials whic are less damaging.
- Use of gases such as Methyl bromide which is a crop fumigant also to be controlled.
- Avoiding the use of halon based fire extinguishers.
- Reducing the usage of automobiles for unnecessary purposes.
References:-
Thanks for ur time and please vote my previous posts

Nice work and nice information from you @saimegh
Good post