Endocannabinoids as Guardians of Metastasis
Abstract
Endocannabinoids including anandamide and 2-arachidonoylglycerol are involved in cancer pathophysiology in several ways, including tumor growth and progression, peritumoral inflammation, nausea and cancer pain. Recently we showed that the endocannabinoid profiles are deranged during cancer to an extent that this manifests in alterations of plasma endocannabinoids in cancer patients, which was mimicked by similar changes in rodent models of local and metastatic cancer. The present topical review summarizes the complexity of endocannabinoid signaling in the context of tumor growth and metastasis.
Keywords: endocannabinoids, anandamide, 2-arachidonoylglycerol, orphan G-protein coupled receptor, immune cells, angiogenesis
- Introduction
Endocannabinoids (eCBs) constitute a growing number of lipid signaling molecules, the most popular being anandamide (AEA) and 2-arachidonoylglycerol (2-AG). They are involved in cancer pathophysiology in several ways (Figure 1), including tumor growth and progression [1], immune (in)tolerance, inflammation [2], nausea [3] and cancer pain [4,5]. Our recent work [6] revealed that the endocannabinoid profiles are deranged during cancer, particularly in metastatic cancer, to an extent that this manifests in alterations of plasma endocannabinoids in cancer patients, which was mimicked by similar changes in rodent models of local and metastatic cancer, suggesting that the monitoring of endocannabinoid profiles might be useful for assessing the individual course of the disease and, possibly, that the derangement of the profiles plays a functional role for cancer progression, potentially giving rise to supportive therapeutic interventions.
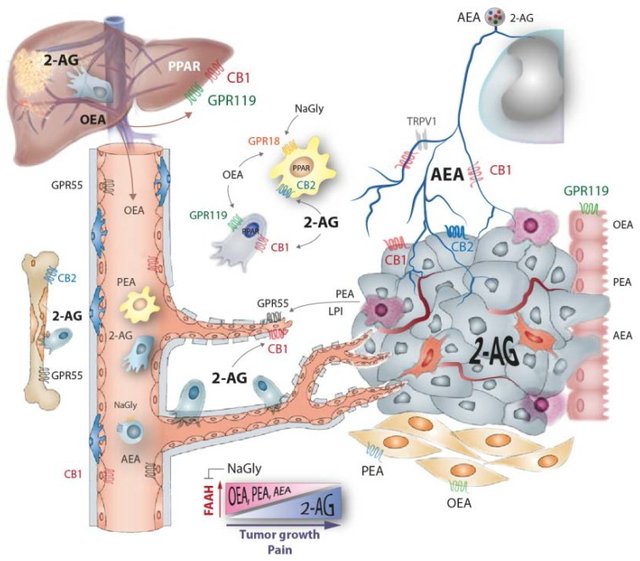
Tumor growth causes an increase of 2-arachidonoylethanolamide (2-AG) in tumor tissue and plasma and a decrease of ethanolamide endocannabinoids anandamide (AEA), oleoylethanolamide (OEA), palmitoylethanolamide (PEA) because the tumor displaces normal ... - Rise of 2-AG in the Tumor Environment and in Plasma
Endocannabinoids are produced in several peripheral tissues resulting in cell-type and location-specific profiles so that the eCB pattern in the tumor microenvironment depends on the tumor’s origin and site of primary growth and metastasis. We showed that tumor growth is associated with an increase of 2-arachidonoylglycerol (2-AG) both at the site of the primary tumor and in plasma [6]. It steadily increased over the course of cancer development and metastasis, suggesting that the growing tumor and circulating metastatic tumor cells secrete large amounts of 2-AG, sufficient enough to manifest in high plasma concentrations. The 2-AG increase is likely contributed by activated immune cells, which are a major source of 2-AG in the periphery at sites of inflammation [7,8]. In the tumor microenvironment 2-AG elicits CB2 receptor signaling of invading immune cells, which may trigger a phenotypic switch from aggressive to tumor-tolerant cells [9] and polarization towards the tumor-helping M2-like macrophages, such as ”tumor associated macrophages” (TAMs) [10]. These TAMs promote tumor invasiveness and metastasis by releasing metalloproteinases and angiogenic factors [11].One of the M2-derived pro-angiogenic factors is palmitoylethanolamide (PEA) which acts as an agonist of endothelial GPR55 receptors [12]. GPR55 is an untypical cannabinoid receptor, which elicits Rho, Rac and CD42 signaling [13], converging on the regulation of cancer and endothelial cell migration [14] and tube formation [15]. GPR55 is also activated by 2-AG-ether, a precursor of 2-AG, which is also known as noladin ether and is likely directly produced and released by the tumor. Further agonists of GPR55 include lysophospholipids, e.g., lysophosphatidylinositol (LPI) [15,16]. These lipids are released by aggregating platelets [17] or produced extracellularly by secretory phospholipases A [18] or D [19]. The latter, also known as autotaxin, attaches to the cell surface of circulating immune or metastatic tumor cells and uses their membrane lipids as precursors for the production of lysophosphatidic acids (LPAs) [20,21], which then stimulate tumor cell migration [22] and angiogenesis via LPA receptors [23]. Vascular cells also express CB1 and GPR18, which mediate vasodilation on agonist binding [24] and thereby increase the tumor’s blood supply - Loss of Ethanolamide Endocannabinoids in Tumor Environment and Plasma
Contrary to 2-AG, we have shown that ethanolamide endocannabinoids (eCBs) anandamide (AEA), oleoylethanolamide (OEA) and palmitoylethanolamide (PEA) decrease in the tumor microenvironment and in plasma [6], likely because the growing tumor displaces normal cells that produce these eCBs and destroys sensory neuronal fibers that are innervating the tumor [33]. These terminals are a major source of AEA in the tumor microenvironment, and as long as these terminals secrete AEA the tumor itself may suppress local cancer pain. The peripheral AEA pool is further contributed by epithelial cells, keratinocytes and muscle cells that all release AEA on demand [34]. AEA is a full agonist of all kinds of cannabinoid receptors that have been identified including CB1, CB2, the orphan GPRs 18, 55, 92 (= LPAR5) and 119, and the nuclear receptors PPAR gamma and alpha [35,36], and transient receptor potential (TRPV1) calcium channels [37]. Most importantly, it acts as an autocrine agonist of CB1 receptors of the peripheral nerves to control nociception [7] and as a CB2 agonist to resolve inflammation. Its decline in the tumor environment is associated with an increase of cancer pain and a deregulation of immune cells.The 2-AG, OEA and PEA have a narrower spectrum of receptors than AEA [38], which also holds true for the exogenous cannabis constituents, tetrahydrocannabinol (THC) and cannabidiol (CBD). Like AEA, both OEA and PEA reciprocally decrease while the tumor mass and metastases increase [6]. All of these eCBs are primarily metabolized by fatty acid amide hydrolase (FAAH) which is upregulated in various types of cancer [25,39], suggesting that enhanced degradation contributes to the loss of production by normal cells. So far, FAAH antagonists have been considered as potential treatments for cancer pain in rodent models [4], but their potential effects on tumor growth and the anti-tumoral immune response have not been assessed. However, interestingly the weak FAAH inhibitor R-flurbiprofen, which leads to a resetting of normal eCB profiles in models of neuropathic pain and autoimmune disease [40,41], was previously shown to reduce tumor growth in transplant models in nude mice [42] and in colon or prostate cancer development in APCmin (Adenomatous Polyposis Coli, multiple intestinal neoplasia) and TRAMP (Transgenic Adenocarcinoma of the Mouse Prostate) mice [43,44]. Although R-flurbiprofen failed in phase II clinical studies of prostate cancer, its positive results may encourage testing of FAAH-based combi-treatments for pain and cancer [45].The primary source of peripheral OEA is the (gut) epithelium, fat and liver [46,47]. It has been mainly considered as a satiety signal, and consequently as a protector against obesity and metabolic syndrome [47,48,49]. Peripheral PEA is likely mainly produced by stromal and immune cells [50]. Both OEA and PEA do not activate the typical CB1 and CB2 cannabinoid receptors, but act through the “orphan” cannabinoid GPRs and PPARs: OEA mainly through GPR119, PPAR gamma and alpha, and PEA mainly through GPR55, GPR18 and PPAR alpha [35,36]. GPR18 and 92 are highly expressed by immune cells including macrophages, T- and B-cells, and the primary full agonist N-arachidonoylglycine, NaGly, a metabolite of AEA [51,52], regulates immune functions and cell migration through these receptors [53,54], suggesting that both may contribute to the fine-tuning of the tumor-evoked immune response. NaGly is produced by many cells and acts as an endogenous inhibitor of FAAH and thereby increases AEA, OEA and PEA levels [55] and counteracts the tumor-mediated loss of these eCBs. - Cannabinoid Receptors of Tumor Cells
Depending on the tumor’s origin, the tumor cells themselves express CB1 and CB2, which has been reviewed elsewhere [56,57,58], and possibly GPR119, the latter particularly in tumors of epithelial origin. Cannabinoid-mediated tumor killing was shown to involve mostly CB1 signaling: one path converging on an increase of ceramides that leads to the endoplasmic reticulum and oxidative stress [1], other pathways converging on Akt, Erk or MAP kinase inhibition [59,60], AMPK-mediated autophagy [61], cell cycle inhibition [62], or still unknown receptors and signaling pathways [63]. The expression of CB1 was identified as a positive prognostic factor for disease-free survival in patients with tongue cancer [64] but not prostate cancer [65], although prostate cancer cells, like other cancer cells, are killed by CB1 or CB2 agonists in vitro [25,66,67,68]. CB2 expression has been recently associated with a poor prognosis in Her2/Neu-positive breast cancer, where its presence promoted pro-oncogenic signaling of Her2 at the level of the tyrosine kinase c-Src [32]. In contract, triple-negative breast cancer cells (estrogen receptor, progesterone receptor and Her2-negative) were killed by a CB2 agonist [69]. It is obvious from multiple studies that the co-expression of the cannabinoid receptor with receptors of the epidermal growth factor receptor (EGFR) or other growth factor families is crucial for the outcome because the formation of heteromers [32] or signaling crosstalk may reverse normal functions. While CB1 and CB2 are well studied in the context of cancer, GPR119 is still an “orphan”, although it is highly expressed in glandular tissue including intestine, pancreas and liver, and its activation leads to lipolysis, insulin secretion and reduction of food intake [70,71]. It is mainly activated by OEA and triggers the OEA-evoked satiety signals [70,71]. - Oleoylethanolamide
OEA concentrations in the plasma of cancer patients were reduced initially but re-raised once local tumor growth turned into metastatic disease [6]. OEA levels were positively associated with the number of metastases in cancer patients, and particularly liver metastases caused an increase of OEA plasma levels, suggesting enhanced secretion from the metastatic liver [6]. To assess the physiologic relevance of OEA in the context of metastasis, we tested its effects in a migration assay in vitro [6]. High OEA concentrations that are not reached in plasma, but in normal tissue surrounding locally restricted tumors, inhibited tumor cell migration. Oppositely, low concentrations enhanced tumor cell proliferation and migration [6], suggesting that the local loss of OEA in the tumor microenvironment facilitates growth and metastasis and the increase of liver OEA secretion may be interpreted as an attempt to stop metastasis. However, despite this metastasis-driven increase, plasma levels remained low compared to those required to stop migration. OEA per se did not qualify as an independent marker of metastasis but might be indicative of individual progression.The concentration-dependent opposing effects of low and high OEA may involve actions through GPR119 and PPARs resulting in opposite pro- or anti-migratory signaling pathways. Besides OEA, anandamide is a strong agonist of both PPAR alpha and gamma whereas 2-AG and PEA mainly act as agonists of PPAR alpha [36]. Tumor cells, immune cells and endothelial cells all express PPARs and both activators and inhibitors were shown to reduce cancer growth or migration [72,73,74]. Hence, by acting through PPARs, all endocannabinoids and exogenous cannabinoids may facilitate or inhibit growth with an unforeseeable outcome. Overall, the net effect of cannabinoid treatment in various models of cancer was a reduction of cancer development and growth [25,26,27,28,29,30,31,59]. However, the opposite was also observed [75,76,77]. The complexity of the endocannabinoid system in the tumor microenvironment of local and metastatic cancer complicates the development of anti-cancer drugs targeting the endogenous cannabinoid system. Nevertheless, FAAH inhibition may be a logical approach to restore normal eCB balances [78,79], whereas inhibition of monoacylglycerol lipase (MAGL) and abhydrolase domain containing 6 (ABHD6) which metabolize 2-AG would instead further shift the balance towards 2-AG. Consequently, both MAGL and ABDH6 inhibition produced a variable outcome [75,80,81], which, however, may depend on the tumor’s origin. - Exogenous Cannabinoids and Therapeutic Implications
The exogenous cannabinoids THC and cannabidiol (CBD) reduced tumor growth in animal models [27,31,82,83,84]. THC acts as an agonist of GPR18, CB1 and CB2 whereas cannabidiol is an antagonist of GPR55, and an agonist of GPR18 and GPR119. Cannabidol does not act through the typical CB1 and CB2 receptors. Hence, the combination of THC with CBD, currently available as oromucosal spray, may favorably combine anti-proliferative CB1-mediated effects and suppression of GPR55-mediated angiogenesis and reduction of cancer pain [85,86], and by acting through GPR18, immune cells may be stimulated to migrate towards and kill tumor cells.THC-mediated activation of CB2-mediated silencing of macrophages may be a disadvantage in terms of the tumor growth, and in certain types of cancer, expression of CB2 was associated with a poor prognosis [32]. On the other hand, CB2 signaling would reduce the surrounding inflammation and, likely, the reduction of cancer pain contributes to a fortification of the immune system, raising the idea of a broader use of cannabinoids in cancer (pain) treatment.However, exogenous cannabinoids cannot replace or restore endogenous cannabinoid profiles, suggesting that drugs targeting the degradation of ethanolamide endocannabinoids may have additional therapeutic value. In addition, the monitoring of individual endocannabinoid profiles over time may be useful for an assessment of disease progression and identification of patients who would likely profit from an eCB-directed therapy.
Acknowledgments
This review was supported by the Deutsche Forschungsgemeinschaft (CRC1039 A3 to IT) and the LOEWE-Center “Translational Medicine & Pharmacology”.
Abbreviations
eCB, endocannabinoid; AEA, anandamide; OEA, oleoylethanolamide; PEA, palmitoylethanolamide; 2-AG, arachidonoylethanolamide; FAAH, fatty acid amide hydrolase; LPI, phosphatidylinositol; NaGly, N-arachidonoylglycine; GPR, G-protein coupled receptor; CB1, CB2, cannabinoid receptor 1 and 2; PPAR, peroxisome proliferator activated receptor; TRPV1, transient receptor potential family V type 1. THC, tetrahydrocannabinol; AMPK, adenosine monophosphate-activated kinase; MAGL, monoacylglycerol lipase; ABHD6, abhydrolase domain containing 6; EGFR epidermal growth factor receptor; Her2/Neu, human epidermal growth factor receptor 2, erb-B2.
Conflicts of Interest
The author declares no conflict of interest.
References - Galve-Roperh I., Sanchez C., Cortes M.L., del Pulgar T.G., Izquierdo M., Guzman M. Anti-tumoral action of cannabinoids: Involvement of sustained ceramide accumulation and extracellular signal-regulated kinase activation. Nat. Med. 2000;6:313–319. [PubMed]
- Di Marzo V., Melck D., de Petrocellis L., Bisogno T. Cannabimimetic fatty acid derivatives in cancer and inflammation. Prostaglandins Lipid Mediat. 2000;61:43–61. doi: 10.1016/S0090-6980(00)00054-X.[PubMed] [Cross Ref]
- Sharkey K.A., Darmani N.A., Parker L.A. Regulation of nausea and vomiting by cannabinoids and the endocannabinoid system. Eur. J. Pharmacol. 2014;722:134–146. doi: 10.1016/j.ejphar.2013.09.068.[PMC free article] [PubMed] [Cross Ref]
- Khasabova I.A., Khasabov S.G., Harding-Rose C., Coicou L.G., Seybold B.A., Lindberg A.E., Steevens C.D., Simone D.A., Seybold V.S. A decrease in anandamide signaling contributes to the maintenance of cutaneous mechanical hyperalgesia in a model of bone cancer pain. J. Neurosci. 2008;28:11141–11152. doi: 10.1523/JNEUROSCI.2847-08.2008. [PMC free article] [PubMed] [Cross Ref]
- Brown I., Cascio M.G., Rotondo D., Pertwee R.G., Heys S.D., Wahle K.W. Cannabinoids and omega-3/6 endocannabinoids as cell death and anticancer modulators. Prog. Lipid Res. 2013;52:80–109. doi: 10.1016/j.plipres.2012.10.001. [PubMed] [Cross Ref]
- Sailler S., Schmitz K., Jaeger E., Ferreiros N., Wicker S., Zschiebsch K., Pickert G., Geisslinger G., Walter C., Tegeder I., et al. Regulation of circulating endocannabinoids associated with cancer and metastases in mice and humans. Oncoscience. 2014;1:272–282. doi: 10.18632/oncoscience.33.[PMC free article] [PubMed] [Cross Ref]
- Agarwal N., Pacher P., Tegeder I., Amaya F., Constantin C.E., Brenner G.J., Rubino T., Michalski C.W., Marsicano G., Monory K., et al. Cannabinoids mediate analgesia largely via peripheral type 1 cannabinoid receptors in nociceptors. Nat. Neurosci. 2007;10:870–879. doi: 10.1038/nn1916. [PMC free article][PubMed] [Cross Ref]
- Centonze D., Battistini L., Maccarrone M. The endocannabinoid system in peripheral lymphocytes as a mirror of neuroinflammatory diseases. Curr. Pharm. Des. 2008;14:2370–2342. doi: 10.2174/138161208785740018. [PubMed] [Cross Ref]
- Pacher P., Ungvari Z. Pleiotropic effects of the CB2 cannabinoid receptor activation on human monocyte migration: Implications for atherosclerosis and inflammatory diseases. Am. J. Physiol. Heart Circ. Physiol. 2008;294:H1133–H1134. doi: 10.1152/ajpheart.00020.2008. [PMC free article] [PubMed] [Cross Ref]
- Tomar S., E E.Z., Nagarkatti M., Nagarkatti P.S. Protective role of cannabinoid receptor 2 activation in galactosamine/lipopolysaccharide-induced acute liver failure through regulation of macrophage polarization and microRNAs. J. Pharmacol. Exp. Ther. 2015;353:369–379. doi: 10.1124/jpet.114.220368.[PMC free article] [PubMed] [Cross Ref]
- Ley S., Weigert A., Heriche J.K., Mille-Baker B., Janssen R.A., Brune B. RNAi screen in apoptotic cancer cell-stimulated human macrophages reveals co-regulation of IL-6/IL-10 expression. Immunobiology. 2013;218:40–51. doi: 10.1016/j.imbio.2012.01.019. [PubMed] [Cross Ref]
- Ryberg E., Larsson N., Sjogren S., Hjorth S., Hermansson N.O., Leonova J., Elebring T., Nilsson K., Drmota T., Greasley P.J. The orphan receptor GPR55 is a novel cannabinoid receptor. Br. J. Pharmacol. 2007;152:1092–1101. doi: 10.1038/sj.bjp.0707460. [PMC free article] [PubMed] [Cross Ref]
- Henstridge C.M., Balenga N.A., Ford L.A., Ross R.A., Waldhoer M., Irving A.J. The GPR55 ligand l-alpha-lysophosphatidylinositol promotes RhoA-dependent Ca2+ signaling and NFAT activation. FASEB J. 2009;23:183–193. doi: 10.1096/fj.08-108670. [PubMed] [Cross Ref]
- Ford L.A., Roelofs A.J., Anavi-Goffer S., Mowat L., Simpson D.G., Irving A.J., Rogers M.J., Rajnicek A.M., Ross R.A. A role for L-alpha-lysophosphatidylinositol and GPR55 in the modulation of migration, orientation and polarization of human breast cancer cells. Br. J. Pharmacol. 2010;160:762–771. doi: 10.1111/j.1476-5381.2010.00743.x. [PMC free article] [PubMed] [Cross Ref]
- Ross R.A. The enigmatic pharmacology of GPR55. Trends Pharmacol. Sci. 2009;30:156–163. doi: 10.1016/j.tips.2008.12.004. [PubMed] [Cross Ref]
- Oka S., Nakajima K., Yamashita A., Kishimoto S., Sugiura T. Identification of GPR55 as a lysophosphatidylinositol receptor. Biochem. Biophys. Res. Commun. 2007;362:928–934. doi: 10.1016/j.bbrc.2007.08.078. [PubMed] [Cross Ref]
- Boucharaba A., Serre C.M., Gres S., Saulnier-Blache J.S., Bordet J.C., Guglielmi J., Clezardin P., Peyruchaud O. Platelet-derived lysophosphatidic acid supports the progression of osteolytic bone metastases in breast cancer. J. Clin. Investig. 2004;114:1714–1725. doi: 10.1172/JCI200422123.[PMC free article] [PubMed] [Cross Ref]
- Fourcade O., Simon M.F., Viode C., Rugani N., Leballe F., Ragab A., Fournie B., Sarda L., Chap H. Secretory phospholipase A2 generates the novel lipid mediator lysophosphatidic acid in membrane microvesicles shed from activated cells. Cell. 1995;80:919–927. doi: 10.1016/0092-8674(95)90295-3.[PubMed] [Cross Ref]
- Peyruchaud O. Novel implications for lysophospholipids, lysophosphatidic acid and sphingosine 1-phosphate, as drug targets in cancer. Anti-Cancer Agents Med. Chem. 2009;9:381–391. doi: 10.2174/1871520610909040381. [PubMed] [Cross Ref]
- Leblanc R., Lee S.C., David M., Bordet J.C., Norman D.D., Patil R., Miller D., Sahay D., Ribeiro J., Clezardin P., et al. Interaction of platelet-derived autotaxin with tumor integrin alphaVbeta3 controls metastasis of breast cancer cells to bone. Blood. 2014;124:3141–3150. doi: 10.1182/blood-2014-04-568683. [PMC free article] [PubMed] [Cross Ref]
- Fulkerson Z., Wu T., Sunkara M., Kooi C.V., Morris A.J., Smyth S.S. Binding of autotaxin to integrins localizes lysophosphatidic acid production to platelets and mammalian cells. J. Biol. Chem. 2011;286:34654–34663. doi: 10.1074/jbc.M111.276725. [PMC free article] [PubMed] [Cross Ref]
- Hama K., Aoki J., Fukaya M., Kishi Y., Sakai T., Suzuki R., Ohta H., Yamori T., Watanabe M., Chun J., et al. Lysophosphatidic acid and autotaxin stimulate cell motility of neoplastic and non-neoplastic cells through LPA1. J. Biol. Chem. 2004;279:17634–17639. doi: 10.1074/jbc.M313927200. [PubMed][Cross Ref]
- Van Meeteren L.A., Ruurs P., Stortelers C., Bouwman P., van Rooijen M.A., Pradere J.P., Pettit T.R., Wakelam M.J., Saulnier-Blache J.S., Mummery C.L., et al. Autotaxin, a secreted lysophospholipase D, is essential for blood vessel formation during development. Mol. Cell. Biol. 2006;26:5015–5022. doi: 10.1128/MCB.02419-05. [PMC free article] [PubMed] [Cross Ref]
- Hiley C.R., Kaup S.S. GPR55 and the vascular receptors for cannabinoids. Br. J. Pharmacol. 2007;152:559–561. doi: 10.1038/sj.bjp.0707421. [PMC free article] [PubMed] [Cross Ref]
- Ligresti A., Bisogno T., Matias I., de Petrocellis L., Cascio M.G., Cosenza V., D’Argenio G., Scaglione G., Bifulco M., Sorrentini I., et al. Possible endocannabinoid control of colorectal cancer growth. Gastroenterology. 2003;125:677–687. doi: 10.1016/S0016-5085(03)00881-3. [PubMed] [Cross Ref]
- Izzo A.A., Aviello G., Petrosino S., Orlando P., Marsicano G., Lutz B., Borrelli F., Capasso R., Nigam S., Capasso F., et al. Increased endocannabinoid levels reduce the development of precancerous lesions in the mouse colon. J. Mol. Med. 2008;86:89–98. doi: 10.1007/s00109-007-0248-4. [PMC free article][PubMed] [Cross Ref]
- Ramer R., Hinz B. Inhibition of cancer cell invasion by cannabinoids via increased expression of tissue inhibitor of matrix metalloproteinases-1. J. Natl. Cancer Inst. 2008;100:59–69. doi: 10.1093/jnci/djm268.[PubMed] [Cross Ref]
- Grimaldi C., Pisanti S., Laezza C., Malfitano A.M., Santoro A., Vitale M., Caruso M.G., Notarnicola M., Iacuzzo I., Portella G., et al. Anandamide inhibits adhesion and migration of breast cancer cells. Exp. Cell Res. 2006;312:363–373. doi: 10.1016/j.yexcr.2005.10.024. [PubMed] [Cross Ref]
- Patsos H.A., Hicks D.J., Dobson R.R., Greenhough A., Woodman N., Lane J.D., Williams A.C., Paraskeva C. The endogenous cannabinoid, anandamide, induces cell death in colorectal carcinoma cells: A possible role for cyclooxygenase 2. Gut. 2005;54:1741–1750. doi: 10.1136/gut.2005.073403.[PMC free article] [PubMed] [Cross Ref]
- Joseph J., Niggemann B., Zaenker K.S., Entschladen F. Anandamide is an endogenous inhibitor for the migration of tumor cells and T lymphocytes. Cancer Immunol. Immunother. 2004;53:723–728. doi: 10.1007/s00262-004-0509-9. [PubMed] [Cross Ref]
- Salazar M., Carracedo A., Salanueva I.J., Hernandez-Tiedra S., Lorente M., Egia A., Vazquez P., Blazquez C., Torres S., Garcia S., et al. Cannabinoid action induces autophagy-mediated cell death through stimulation of ER stress in human glioma cells. J. Clin. Investig. 2009;119:1359–1372. doi: 10.1172/JCI37948. [PMC free article] [PubMed] [Cross Ref]
- Perez-Gomez E., Andradas C., Blasco-Benito S., Caffarel M.M., Garcia-Taboada E., Villa-Morales M., Moreno E., Hamann S., Martin-Villar E., Flores J.M., et al. Role of cannabinoid receptor CB2 in HER2 pro-oncogenic signaling in breast cancer. J. Natl. Cancer Inst. 2015;107:djv077. doi: 10.1093/jnci/djv077.[PubMed] [Cross Ref]
- Constantin C.E., Mair N., Sailer C.A., Andratsch M., Xu Z.Z., Blumer M.J., Scherbakov N., Davis J.B., Bluethmann H., Ji R.R., et al. Endogenous tumor necrosis factor alpha (TNFalpha) requires TNF receptor type 2 to generate heat hyperalgesia in a mouse cancer model. J. Neurosci. 2008;28:5072–5081. doi: 10.1523/JNEUROSCI.4476-07.2008. [PubMed] [Cross Ref]
- Heyman E., Gamelin F.X., Goekint M., Piscitelli F., Roelands B., Leclair E., di Marzo V., Meeusen R. Intense exercise increases circulating endocannabinoid and BDNF levels in humans-Possible implications for reward and depression. Psychoneuroendocrinology. 2012;37:844–851. doi: 10.1016/j.psyneuen.2011.09.017. [PubMed] [Cross Ref]
- Yin H., Chu A., Li W., Wang B., Shelton F., Otero F., Nguyen D.G., Caldwell J.S., Chen Y.A. Lipid G protein-coupled receptor ligand identification using beta-arrestin PathHunter assay. J. Biol. Chem. 2009;284:12328–12338. doi: 10.1074/jbc.M806516200. [PMC free article] [PubMed] [Cross Ref]
- O’Sullivan S.E. Cannabinoids go nuclear: Evidence for activation of peroxisome proliferator-activated receptors. Br. J. Pharmacol. 2007;152:576–582. doi: 10.1038/sj.bjp.0707423. [PMC free article] [PubMed][Cross Ref]
- Van der Stelt M., Trevisani M., Vellani V., de Petrocellis L., Schiano Moriello A., Campi B., McNaughton P., Geppetti P., di Marzo V. Anandamide acts as an intracellular messenger amplifying Ca2+influx via TRPV1 channels. Embo J. 2005;24:3026–3037. doi: 10.1038/sj.emboj.7600784.[PMC free article] [PubMed] [Cross Ref]
- Bradshaw H.B., Lee S.H., McHugh D. Orphan endogenous lipids and orphan GPCRs: A good match. Prostaglandins Lipid Mediat. 2009;89:131–134. doi: 10.1016/j.prostaglandins.2009.04.006.[PMC free article] [PubMed] [Cross Ref]
- Endsley M.P., Thill R., Choudhry I., Williams C.L., Kajdacsy-Balla A., Campbell W.B., Nithipatikom K. Expression and function of fatty acid amide hydrolase in prostate cancer. Int. J. Cancer. 2008;123:1318–1326. doi: 10.1002/ijc.23674. [PMC free article] [PubMed] [Cross Ref]
- Bishay P., Schmidt H., Marian C., Haussler A., Wijnvoord N., Ziebell S., Metzner J., Koch M., Myrczek T., Bechmann I., et al. R-flurbiprofen reduces neuropathic pain in rodents by restoring endogenous cannabinoids. PLoS ONE. 2010;5:230 doi: 10.1371/journal.pone.0010628. [PMC free article][PubMed] [Cross Ref]
- Schmitz K., de Bruin N., Bishay P., Mannich J., Haussler A., Altmann C., Ferreiros N., Lotsch J., Ultsch A., Parnham M.J., et al. R-flurbiprofen attenuates experimental autoimmune encephalomyelitis in mice. EMBO Mol. Med. 2014;6:1398–13422. doi: 10.15252/emmm.201404168. [PMC free article][PubMed] [Cross Ref]
- Grosch S., Tegeder I., Schilling K., Maier T.J., Niederberger E., Geisslinger G. Activation of c-Jun-N-terminal-kinase is crucial for the induction of a cell cycle arrest in human colon carcinoma cells caused by flurbiprofen enantiomers. FASEB J. 2003;17:1316–1318. doi: 10.1096/fj.02-0919fje. [PubMed][Cross Ref]
- Wechter W.J., Leipold D.D., Murray E.D., Jr., Quiggle D., McCracken J.D., Barrios R.S., Greenberg N.M. E-7869 (R-flurbiprofen) inhibits progression of prostate cancer in the TRAMP mouse. Cancer Res. 2000;60:2203–2208. [PubMed]
- Wechter W.J., Kantoci D., Murray E.D., Jr., Quiggle D.D., Leipold D.D., Gibson K.M., McCracken J.D. R-flurbiprofen chemoprevention and treatment of intestinal adenomas in the APC(Min)/+ mouse model: Implications for prophylaxis and treatment of colon cancer. Cancer Res. 1997;57:4316–4324.[PubMed]
- Fowler C.J. Possible involvement of the endocannabinoid system in the actions of three clinically used drugs. Trends Pharmacol. Sci. 2004;25:59–61. doi: 10.1016/j.tips.2003.12.001. [PubMed] [Cross Ref]
- Guzman M., Lo Verme J., Fu J., Oveisi F., Blazquez C., Piomelli D. Oleoylethanolamide stimulates lipolysis by activating the nuclear receptor peroxisome proliferator-activated receptor alpha (PPAR-alpha) J. Biol. Chem. 2004;279:27849–27854. doi: 10.1074/jbc.M404087200. [PubMed] [Cross Ref]
- Schwartz G.J., Fu J., Astarita G., Li X., Gaetani S., Campolongo P., Cuomo V., Piomelli D. The lipid messenger OEA links dietary fat intake to satiety. Cell Metab. 2008;8:281–288. doi: 10.1016/j.cmet.2008.08.005. [PMC free article] [PubMed] [Cross Ref]
- Rodriguez de Fonseca F., Navarro M., Gomez R., Escuredo L., Nava F., Fu J., Murillo-Rodriguez E., Giuffrida A., LoVerme J., Gaetani S., et al. An anorexic lipid mediator regulated by feeding. Nature. 2001;414:209–212. doi: 10.1038/35102582. [PubMed] [Cross Ref]
- Soria-Gomez E., Guzman K., Pech-Rueda O., Montes-Rodriguez C.J., Cisneros M., Prospero-Garcia O. Oleoylethanolamide affects food intake and sleep-waking cycle through a hypothalamic modulation. Pharmacol. Res. 2010;61:379–384. doi: 10.1016/j.phrs.2010.01.010. [PubMed] [Cross Ref]
- Parolaro D., Massi P., Rubino T., Monti E. Endocannabinoids in the immune system and cancer. Prostaglandins Leukot. Essent. Fat. Acids. 2002;66:319–332. doi: 10.1054/plef.2001.0355. [PubMed][Cross Ref]
- Bradshaw H.B., Rimmerman N., Hu S.S., Benton V.M., Stuart J.M., Masuda K., Cravatt B.F., O’Dell D.K., Walker J.M. The endocannabinoid anandamide is a precursor for the signaling lipid N-arachidonyl glycine through two distinct pathways. BMC Biochem. 2009;10:14. doi: 10.1186/1471-2091-10-14.[PMC free article] [PubMed] [Cross Ref]
- Burstein S.H., Huang S.M., Petros T.J., Rossetti R.G., Walker J.M., Zurier R.B. Regulation of anandamide tissue levels by N-arachidonylglycine. Biochem. Pharmacol. 2002;64:1147–1150. doi: 10.1016/S0006-2952(02)01301-1. [PubMed] [Cross Ref]
- McHugh D., Wager-Miller J., Page J., Bradshaw H.B. siRNA knockdown of GPR18 receptors in BV-2 microglia attenuates N-arachidonoyl glycine-induced cell migration. J. Mol. Signal. 2012;7:10. doi: 10.1186/1750-2187-7-10. [PMC free article] [PubMed] [Cross Ref]
- McHugh D., Page J., Dunn E., Bradshaw H.B. Delta(9)-Tetrahydrocannabinol and N-arachidonyl glycine are full agonists at GPR18 receptors and induce migration in human endometrial HEC-1B cells. Br. J. Pharmacol. 2012;165:2414–2424. doi: 10.1111/j.1476-5381.2011.01497.x. [PMC free article] [PubMed][Cross Ref]
- Grazia Cascio M., Minassi A., Ligresti A., Appendino G., Burstein S., Di Marzo V. A structure-activity relationship study on N-arachidonoyl-amino acids as possible endogenous inhibitors of fatty acid amide hydrolase. Biochem. Biophys. Res. Commun. 2004;314:192–196. doi: 10.1016/j.bbrc.2003.12.075.[PubMed] [Cross Ref]
- Velasco G., Sanchez C., Guzman M. Endocannabinoids and Cancer. Handb. Exp. Pharmacol. 2015;231:449–472. [PubMed]
- Pisanti S., Picardi P., D’Alessandro A., Laezza C., Bifulco M. The endocannabinoid signaling system in cancer. Trends Pharmacol. Sci. 2013;34:273–282. doi: 10.1016/j.tips.2013.03.003. [PubMed] [Cross Ref]
- Chakravarti B., Ravi J., Ganju R.K. Cannabinoids as therapeutic agents in cancer: Current status and future implications. Oncotarget. 2014;5:5852–5872. doi: 10.18632/oncotarget.2233. [PMC free article][PubMed] [Cross Ref]
- Bifulco M., Laezza C., Gazzerro P., Pentimalli F. Endocannabinoids as emerging suppressors of angiogenesis and tumor invasion (review) Oncol. Rep. 2007;17:813–816. doi: 10.3892/or.17.4.813.[PubMed] [Cross Ref]
- Caffarel M.M., Andradas C., Mira E., Perez-Gomez E., Cerutti C., Moreno-Bueno G., Flores J.M., Garcia-Real I., Palacios J., Manes S., et al. Cannabinoids reduce ErbB2-driven breast cancer progression through Akt inhibition. Mol. Cancer. 2010;9:196. doi: 10.1186/1476-4598-9-196. [PMC free article][PubMed] [Cross Ref]
- Vara D., Salazar M., Olea-Herrero N., Guzman M., Velasco G., Diaz-Laviada I. Anti-tumoral action of cannabinoids on hepatocellular carcinoma: Role of AMPK-dependent activation of autophagy. Cell Death Differ. 2011;18:1099–1111. doi: 10.1038/cdd.2011.32. [PMC free article] [PubMed] [Cross Ref]
- Laezza C., Pisanti S., Crescenzi E., Bifulco M. Anandamide inhibits Cdk2 and activates Chk1 leading to cell cycle arrest in human breast cancer cells. FEBS Lett. 2006;580:6076–6082. doi: 10.1016/j.febslet.2006.09.074. [PubMed] [Cross Ref]
- Gustafsson S.B., Lindgren T., Jonsson M., Jacobsson S.O. Cannabinoid receptor-independent cytotoxic effects of cannabinoids in human colorectal carcinoma cells: Synergism with 5-fluorouracil. Cancer Chemother. Pharmacol. 2009;63:691–701. doi: 10.1007/s00280-008-0788-5. [PubMed] [Cross Ref]
- Theocharis S., Giaginis C., Alexandrou P., Rodriguez J., Tasoulas J., Danas E., Patsouris E., Klijanienko J. Evaluation of cannabinoid CB1 and CB2 receptors expression in mobile tongue squamous cell carcinoma: Associations with clinicopathological parameters and patients’ survival. Tumour Biol. 2015:1–10. doi: 10.1007/s13277-015-4182-8. [PubMed] [Cross Ref]
- Chung S.C., Hammarsten P., Josefsson A., Stattin P., Granfors T., Egevad L., Mancini G., Lutz B., Bergh A., Fowler C.J. A high cannabinoid CB(1) receptor immunoreactivity is associated with disease severity and outcome in prostate cancer. Eur. J. Cancer. 2009;45:174–182. doi: 10.1016/j.ejca.2008.10.010.[PubMed] [Cross Ref]
- Orellana-Serradell O., Poblete C.E., Sanchez C., Castellon E.A., Gallegos I., Huidobro C., Llanos M.N., Contreras H.R. Proapoptotic effect of endocannabinoids in prostate cancer cells. Oncol. Rep. 2015;33:1599–1608. doi: 10.3892/or.2015.3746. [PMC free article] [PubMed] [Cross Ref]
- Preet A., Qamri Z., Nasser M.W., Prasad A., Shilo K., Zou X., Groopman J.E., Ganju R.K. Cannabinoid receptors, CB1 and CB2, as novel targets for inhibition of non-small cell lung cancer growth and metastasis. Cancer Prev. Res. 2011;4:65–75. [PMC free article] [PubMed]
- Qamri Z., Preet A., Nasser M.W., Bass C.E., Leone G., Barsky S.H., Ganju R.K. Synthetic cannabinoid receptor agonists inhibit tumor growth and metastasis of breast cancer. Mol. Cancer Ther. 2009;8:3117–3329. [PMC free article] [PubMed]
- Morales P., Blasco-Benito S., Andradas C., Gomez-Canas M., Flores J.M., Goya P., Fernandez-Ruiz J., Sanchez C., Jagerovic N. Selective, nontoxic CB(2) cannabinoid o-quinone with in vivo activity against triple-negative breast cancer. J. Med. Chem. 2015;58:2256–2264. doi: 10.1021/acs.jmedchem.5b00078.[PubMed] [Cross Ref]
- Overton H.A., Babbs A.J., Doel S.M., Fyfe M.C., Gardner L.S., Griffin G., Jackson H.C., Procter M.J., Rasamison C.M., Tang-Christensen M., et al. Deorphanization of a G protein-coupled receptor for oleoylethanolamide and its use in the discovery of small-molecule hypophagic agents. Cell Metab. 2006;3:167–175. [PubMed]
- Godlewski G., Offertaler L., Wagner J.A., Kunos G. Receptors for acylethanolamides-GPR55 and GPR119. Prostaglandins Lipid Mediat. 2009;89:105–111. [PMC free article] [PubMed]
- Pang X., Wei Y., Zhang Y., Zhang M., Lu Y., Shen P. Peroxisome proliferator-activated receptor-gamma activation inhibits hepatocellular carcinoma cell invasion by upregulating plasminogen activator inhibitor-1. Cancer Sci. 2013;104:672–680. doi: 10.1111/cas.12143. [PubMed] [Cross Ref]
- Apostoli A.J., Skelhorne-Gross G.E., Rubino R.E., Peterson N.T., Di Lena M.A., Schneider M.M., Sengupta S.K., Nicol C.J. Loss of PPARgamma expression in mammary secretory epithelial cells creates a pro-breast tumorigenic environment. Int. J. Cancer. 2013;134:1055–1066. doi: 10.1002/ijc.28432.[PMC free article] [PubMed] [Cross Ref]
- Pignatelli M., Cortes-Canteli M., Lai C., Santos A., Perez-Castillo A. The peroxisome proliferator-activated receptor gamma is an inhibitor of ErbBs activity in human breast cancer cells. J. Cell Sci. 2001;114:4117–4126. [PubMed]
- Nomura D.K., Lombardi D.P., Chang J.W., Niessen S., Ward A.M., Long J.Z., Hoover H.H., Cravatt B.F. Monoacylglycerol lipase exerts dual control over endocannabinoid and fatty acid pathways to support prostate cancer. Chem. Biol. 2011;18:846–856. doi: 10.1016/j.chembiol.2011.05.009. [PMC free article][PubMed] [Cross Ref]
- Hu W.R., Lian Y.F., Peng L.X., Lei J.J., Deng C.C., Xu M., Feng Q.S., Chen L.Z., Bei J.X., Zeng Y.X. Monoacylglycerol lipase promotes metastases in nasopharyngeal carcinoma. Int. J. Clin. Exp. Pathol. 2014;7:3704–3713. [PMC free article] [PubMed]
- Takeda S., Yamaori S., Motoya E., Matsunaga T., Kimura T., Yamamoto I., Watanabe K. Delta(9)-Tetrahydrocannabinol enhances MCF-7 cell proliferation via cannabinoid receptor-independent signaling. Toxicology. 2008;245:141–146. doi: 10.1016/j.tox.2007.12.019. [PubMed] [Cross Ref]
- Di Marzo V., Melck D., Orlando P., Bisogno T., Zagoory O., Bifulco M., Vogel Z., de Petrocellis L. Palmitoylethanolamide inhibits the expression of fatty acid amide hydrolase and enhances the anti-proliferative effect of anandamide in human breast cancer cells. Biochem. J. 2001;358:249–255. doi: 10.1042/bj3580249. [PMC free article] [PubMed] [Cross Ref]
- Thors L., Bergh A., Persson E., Hammarsten P., Stattin P., Egevad L., Granfors T., Fowler C.J. Fatty acid amide hydrolase in prostate cancer: Association with disease severity and outcome, CB1 receptor expression and regulation by IL-4. PLoS ONE. 2010;5:230 doi: 10.1371/journal.pone.0012275.[PMC free article] [PubMed] [Cross Ref]
- Nomura D.K., Long J.Z., Niessen S., Hoover H.S., Ng S.W., Cravatt B.F. Monoacylglycerol lipase regulates a fatty acid network that promotes cancer pathogenesis. Cell. 2010;140:49–61. doi: 10.1016/j.cell.2009.11.027. [PMC free article] [PubMed] [Cross Ref]
- Sun H., Jiang L., Luo X., Jin W., He Q., An J., Lui K., Shi J., Rong R., Su W., et al. Potential tumor-suppressive role of monoglyceride lipase in human colorectal cancer. Oncogene. 2013;32:234–241. doi: 10.1038/onc.2012.34. [PMC free article] [PubMed] [Cross Ref]
- Fowler C.J. Delta(9)-tetrahydrocannabinol and cannabidiol as potential curative agents for cancer: A critical examination of the preclinical literature. Clin. Pharmacol. Ther. 2015;97:587–596. doi: 10.1002/cpt.84. [PubMed] [Cross Ref]
- Torres S., Lorente M., Rodriguez-Fornes F., Hernandez-Tiedra S., Salazar M., Garcia-Taboada E., Barcia J., Guzman M., Velasco G. A combined preclinical therapy of cannabinoids and temozolomide against glioma. Mol. Cancer Ther. 2011;10:90–103. doi: 10.1158/1535-7163.MCT-10-0688. [PubMed][Cross Ref]
- Hernan Perez de la Ossa D., Lorente M., Gil-Alegre M.E., Torres S., Garcia-Taboada E., Aberturas Mdel R., Molpeceres J., Velasco G., Torres-Suarez A.I. Local delivery of cannabinoid-loaded microparticles inhibits tumor growth in a murine xenograft model of glioblastoma multiforme. PLoS ONE. 2013;8:230 doi: 10.1371/journal.pone.0054795. [PMC free article] [PubMed] [Cross Ref]
- Armstrong J.L., Hill D.S., McKee C.S., Hernandez-Tiedra S., Lorente M., Lopez-Valero I., Eleni Anagnostou M., Babatunde F., Corazzari M., Redfern C.P., et al. Exploiting cannabinoid-induced cytotoxic autophagy to drive melanoma cell death. J. Investig. Dermatol. 2015;135:1629–1637. doi: 10.1038/jid.2015.45. [PubMed] [Cross Ref]
- Johnson J.R., Lossignol D., Burnell-Nugent M., Fallon M.T. An open-label extension study to investigate the long-term safety and tolerability of THC/CBD oromucosal spray and oromucosal THC spray in patients with terminal cancer-related pain refractory to strong opioid analgesics. J. Pain Symptom Manag. 2013;46:207–218. doi: 10.1016/j.jpainsymman.2012.07.014. [PubMed] [Cross Ref]
Source: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4783962/
Don't forget to upvote If you like my post
Nice post! Good to see someone rocking the science 🖤
Thats true @blackmetalchem , enjoy!
Wow! Just...wow. Thank you.
X
Thank you to @xmaas