Answer: What are some statistics that show increased risks after getting the covid vaccines? (Part 1)
Increased risks of what? If you mean SAEs or AESIs, depending on how the literature classifies them, there are thousands of studies documenting them, but I can only cover the tip of the iceberg within a Quora answer that limits the amount of literature I can reasonably summarize.
BMJ reanalysis of phase III trial data:An analysis of the phase 3 trial data showed that, while rare, there was a statistically significant greater incidence of serious adverse events in both the Pfizer and Moderna treatment groups than in both placebo groups. In fact, the researchers found a much greater disparity in serious adverse events than what had been reported by the FDA, noting that the FDA included thousands of participants who had only received one dose of Pfizer, with very few follow-ups, and a median follow up time of 1 month after the shot, while they limited their analysis to only participants who had received both Pfizer shots and a median follow-up time of 2 months.
The FDA reported 126 of 21,621 (0.6 %) of vaccinated participants experienced at least one SAE at data cutoff compared to 111 of 21,631 (0.5 %) of placebo participants. In contrast, our analysis found 127 SAEs among 18,801 vaccine recipients versus 93 SAEs among 18,785 placebo recipients. [15] While summary results for the population we analyzed was provided in a table, FDA did not report an analysis of them. The substantially larger denominators in FDA’s analysis (5,666 more participants) reflect the fact that their analysis included all individuals receiving at least one dose (minus 196 HIV-positive participants), irrespective of the duration of post-injection follow-up time. In contrast, our analysis was based on the study population with median follow-up ≥ 2 months after dose 2 (minus 120 HIV-positive participants), of which 98.1 % had received both doses. [2], [17] The FDA’s analysis of SAEs thus included thousands of additional participants with very little follow-up, of which the large majority had only received 1 dose.
This resulted in finding a 36% higher risk of a serious adverse events in the Pfizer treatment group compared to the placebo group. Risk of AESI for the Pfizer shot is about 12.5 excess SAEs per 10,000 recipients or 1 in 800 over the placebo baseline. This is not stratified by age, sex or health since Pfizer has still yet to release participant level data.
Self-reported Moderna data on all Adverse Events between December 2020 and December 2022 reveals that out of the 772,908,958 doses administered globally over two years there were 418,715 SAEs (1 SAE per 1,846 mRNA1237 doses). The Pharmacovigilance study defined SAEs as adverse events resulting in ‘death, hospitalization, disability/permanent damage, or other events jeopardizing the patient that might require medical/surgical intervention.’ 17,751 SAEs were fatal (1 fatality per 43,542 mRNA1237 doses) with COVID19 only accounting for 3.9% of deaths.
The Full Spectrum of Shot Induced Autoimmune Disorders
Tan et al., conducted a systematic review of 132 studies of fatal AEs including Anaphylaxis (n = 33), myocarditis (n = 32), and Thrombosis (n = 67) with 43 cohort studies and 89 case studies. The studies of shot induced anaphylaxis included 25 cohort studies and 8 case reports. An incident rate between 1 case per 12,500 doses to 1 case per 200 doses of Pfizer was extrapolated with 1,151 total cases. An incident rate between 1 case per 50,000 doses to 1 case per 100 Moderna doses was extrapolated from 544 cases. An incident rate between 1 case per 10,000 doses to 1 case per 333.3 AstraZeneca-Oxford doses was extrapolated from 875 cases. Three studies link severe and fatal allergic reactions to Pfizer doses to the use of polyethylene glycol in the LNPs to increase the half life of the vector which is still very short lived. The package inserts for Comirnaty shot even includes this warning:
Do not administer Pfizer-BioNTech COVID-19 Vaccine to individuals with a history of a severe allergic reaction (e.g., anaphylaxis) to any component of the Pfizer-BioNTech COVID-19 Vaccine [see Description (11)] or to individuals who had a severe allergic reaction (e.g., anaphylaxis) following a previous dose of a Pfizer-BioNTech COVID-19 vaccine.
Just going off the U.S. the baseline rate of anaphylaxis is between 1.6% and 5% of the general population but even that lower figure is over 5 million people which is close to the number of reports in VAERS.
The studies of shot induced myocarditis included 8 cohort studies and 24 case reports. A myocarditis incident rate between 1 case per 250,000 to 1 case per 333.3 Pfizer doses was extrapolated with 1,059 total cases. A myocarditis incident rate between 1 case per 5,000 doses to 1 case per 1,000 Moderna doses was extrapolated from 249 cases. A myocarditis incident rate of 1 case per 25,000 doses was found for the AstraZeneca-Oxford primary series from 178 cases and an incident rate of 1 case per 5,000 doses was found for the Janssen primary series from 8 cases. 80% of cases occur after the second dose and males between the ages of 12-17 and 18-24 years of age have the highest risk of shot induced myocarditis.
The studies of shot induced Thrombosis (blood clots) included 10 cohort studies and 57 case studies that recorded a total of 24,000 cases of induced blood clots. An incident rate between 1 case per 20,000 doses and 1 case per 167 doses was found for the AstraZeneca-Oxford primary series which contributed to most of the blood clot case load. The Janssen primary series was found to lead to blood clots at an incident rate of between 1 case per 125,000 doses and 1 case per 33,333 doses while the blood clot incident rate for Pfizer was between 1 case per 167,000 doses and as high as 1 case per 1,000 doses. The Incident rate of blood clots from the Moderna shots was found to be negligible at about 1 case per 2.5 million doses. The review found 238 deaths related to blood clots caused by Pfizer doses, 186 deaths related to blood clots caused by AstraZeneca Oxford doses, 54 deaths due to Moderna doses and only 17 caused by Janssen doses. A year after vaccine roll out, a review of Thromboembolic adverse events following administration of Janssen doses identified 3,790 reports of shot induced thrombosis, of which 2,892 were serious adverse events requiring hospitalization and of which 421 deaths occurred.
A retrospective cohort study published in Drugs Real World Outcomes combed through 33 million SAEs reported to WHO’s Vigibase to compare the reporting odds ratio (ROR) of each SAE following boosting with 5 different brands included the more recent Novavax. Table 3 shows that while Pfizer (16.85) and Moderna (7.57) carry a much higher relative risk of myocarditis compared to Novavax (5.21) or either adenoviral vector (1.87 and 0.57) Novavax carries a higher risk of pericarditis (24.75) than either modRNA or adenoviral vector platform. Stratified by age and sex, males 18-44 years of age carry the highest risk of myocarditis, consistent with most literature on the matter, and likely it is males between 18-29 years of age who would suffer 1.5-4.6 cases of booster induced myocarditis and pericarditis for every omicron hospitalization theoretically averted by boosting; and as I noted in (Part 7) 76% of such cases result in hospitalization themselves . As I mentioned in a prior post Novavax has an average 28% VE against infection 3-4 months after administration which while abysmal is much more stable than either modRNA, but even this generous estimate might not hold true as the much more immune evasive HV1 becomes the dominate sub variant in the US while boosters are stuck immunizing against XBB1.5.
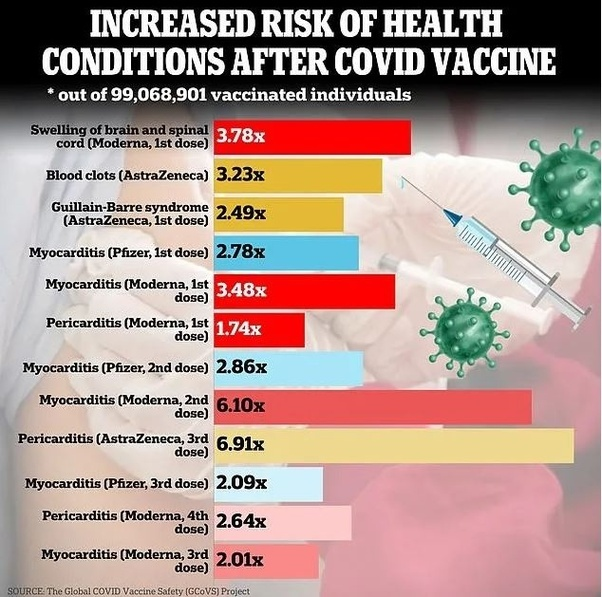
A multi-country retrospective cohort study, published in Vaccine, that analyzed and compared the pre-COVID background and observed rates of 13 AESI using the Adverse Events of Special Interest Study Protocol, to analyze and compare data from 10 sites across 8 countries (n = 99 million), from December 2020 to August 2023 found that 5 AESI occurred frequently enough over the baseline rates in the general population that they exceed the threshold for prioritized vaccine safety signals. These 5 AESI, which exceeded an observed to expected event ratio at the lower bound (of the confidence interval) greater than 1.5 included Guillain-Barré syndrome following the first Astrazeneca dose (2.49), cerebral venous sinus thrombosis following the first Astrazeneca dose (3.23), Acute disseminated encephalomyelitis following the first Moderna dose (3.78), myocarditis following the first 3 Moderna and Pfizer doses (6.10), and pericarditis following the Moderna primary series (2.64) and booster and Astrazeneca booster (6.91) within 42 days of the last dose.
A cross sectional survey study of adult modRNA recipients residing in Saudi Arabia who received at least one dose (n = 804) found that 27.11% of participants (n = 218) reported being diagnosed with cardiac complications post modRNA therapy dose and being hospitalized for such complications.
A nationwide retrospective cohort study (n = 2.2 million) drawing on data from the Korean National Health Insurance Service (NHIS) found that covid shot recipients were 2-5x more likely to develop musculoskeletal disorders including plantar heel pain, rotator cuff syndrome, frozen shoulder, and spinal disc herniation compared to the unvaccinated cohort (n = 336,00).
Another nationwide Korean study (n = 2.2 million), using the same National Health Insurance Service database, found that covid shot recipients were 5.7x more likely to develop coagulation defects and about 2x more likely to develop bone marrow aplasia.
Another nationwide population-based cohort study in S. Korea (n = 2.43 million) found that 2.2% of shot recipients developed vitiligo 3 months after administration compared to only 0.6% of unvaccinated residents with a higher incident rate in females than males and recipients of two or more doses. The incident of all autoimmune disorders in the unvaccinated cohort was found to be comparable to the baseline rate for the pre-pandemic general population and SARS-COV-2 infections were not associated with vitiligo.
In a Trialsite Oped, Dr. Ronald N. Kostoff used the VAERS database to identify 448,520 adverse events related to skin disorders following vaccination that had 765 symptoms listed in the Medical Dictionary for Regulatory Activities.
Another retrospective population-based cohort study of individuals with a history of uveitis (n = 473,934) found that the cumulative incident of uveitis recurrence doubled from 8.6% 3 months after the last dose to 16.8% a year after their last dose.
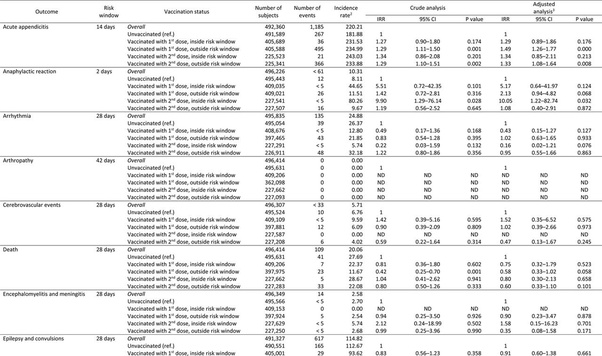
A nationwide retrospective cohort study of adolescents in Norway (n = 496,432), born between 2002-09, found that the first dose of the primary series was associated with a 2x increase in incident rate of anaphylactic shock, outside the 2 day risk window, while the second dose was associated with a 10x increase in the incident of anaphylactic shock inside the 2 day risk window. The second dose was also associated with a 2.3x increase in the incident of lymph nodes swelling and a 5.25x increase in myocarditis and pericarditis and a 1.6x increase in the incidence of epileptic convulsions. The study also found a slightly higher incidence of death, inside the 28 day risk window, following the second dose of the primary series (IRR =1.04).
A Turkish rodent study (n = 41), published in Neurochemical Research, which consisted of both a behavioral assessment and a biochemical evaluation of wistar rats exposed to the Pfizer-Bio-N-Tech shot or saline placebo pre-birth (n = 15), found sex specific motor performance deficits in male rats, exposed to Pfizer-Bio-N-Tech shot during gestation, and (statistically) significant decrease in neural counts in their hippocampus.