NOx Emission and Control in Cement Industry
Nitrogen oxides (NOx) are gaseous pollutants that are primarily formed through combustion process. While flue gas is within the combustion unit, about 95% of the NOx exists in the form of nitric oxide (NO). The balance is nitrogen dioxide (NO2), which is unstable at high temperatures. Once the flue gas is emitted into the atmosphere, most of the NOx is ultimately converted to NO2. NOx in the atmosphere reacts in the presence of sunlight to form ozone (O3), one of the criteria pollutants for which health-based National Ambient Air Quality Standards have been established.
HOW NOx EFFECT US:
• Air is mixture of different gases, mainly 79% N2 and 21% O2 (N2 is remain inert in normal temperature).
• NOx is formed during high temperature combustion in presence of excess O2. NOx emission contributes to the formation of fine particles and ozone smog that cost society billions of dollars annually from illnesses and deaths.
· Nitrogen oxides eventually form nitric acid when dissolved in atmospheric moisture, forming a component of acid rain.
This chemical reaction occurs when nitrogen dioxide reacts with water:
2NO2 + H2O → HNO2 + HNO3
Calculation of NOx Emission:
Conversion at 10% O2
Given: 500 ppm NOx @ 4% O2
500 ppm(21-10)/(21-4) = 323 @10%O2
323@10%O2 = 3232.054 = 663.4 mg/Nm3 at 10% O2
Measuring Unit
NOx Emission: 1 ppm NOx = 2.054 mg NOx/Nm3 (as NO2)
NOx SOURCES:
- Feed NOx: Very Minor to no contribution
- Thermal NOx: Created by reaction of N2 and O2 in air at temperature of >1200 °C (i.e. – Kiln)
- Fuel NOx: Formed in Calciner by combustion of Nitrogen Atom in fuel
Thermal NOx Formation
• NOx formed in the high-temperature environment in the burning zone of a kiln is “thermal NOx” at the range 1200-1600°C. Thermal NOx is formed by oxidation of atmospheric nitrogen at high temperatures.
• Since the flame temperature in a kiln is significantly above these temperatures, considerable amounts of thermal NO are generated in the burning zone. The thermal reaction between oxygen and nitrogen is simplified as follows:
O + N2 → NO + N
N + O2 → NO + O
• NO formation increases exponentially as temperature increases, and increases as excess oxygen (O2) increases. Above 1400°C, small changes in temperature produce large changes in concentrations of NO at a given oxygen concentration.
• Gas temperatures in kiln burning zones are significantly above clinker material temperatures, which must reach about 1450°C to form some clinker compounds.
Fuel NOx Formation
• NOx can also result from oxidation of nitrogen compounds in fuel (fuel NOx).
• Fuel nitrogen is only partially converted into NO during combustion and this reaction occurs throughout the temperature range of the combustion process relatively independent of temperature.
NOx formation in the calciner:
"N" + O → NO 1
"N" + NO → N2 + O 2
AT 800-1000 °C
Reaction (2) is highly effective by temperature. If temperature rises in the system NOx increases accordingly.
NOx Control Strategy:
- Process Optimization
- Hardware Changes
- Selective Non-Catalytic Reduction (SNCR) System
Process Optimization:
a. Nitrogen Content in the fuel
b. O2 level in the firing Zone
c. Initial NOx concentration in the combustion gas
d. Volatile content in solid fuel
e. Temperature in secondary firing zone
f. Optimization of Primary and conveying air at Kiln Burner
g. Kiln inlet O2 control
h. Burning zone temperature
i. Monitoring free lime
NOx Control
· Conversion from direct-fired coal systems to indirect systems to reduce primary air quantity.
· Installation of a new burner (“low NOx” burner)
· Reduction in the amount of excess air (oxygen) used for combustion.
· Split the feed to calciner to create the hot Zone in the calciner immediately at coal firing point.
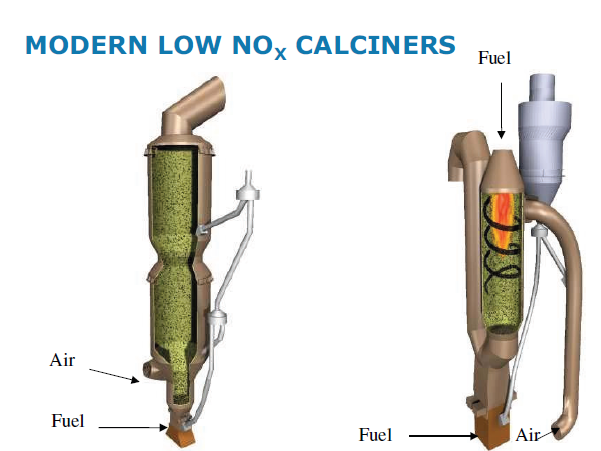
· Use “Low – NOx calciners”. Calciner retrofit is possible for In Line Calciners.
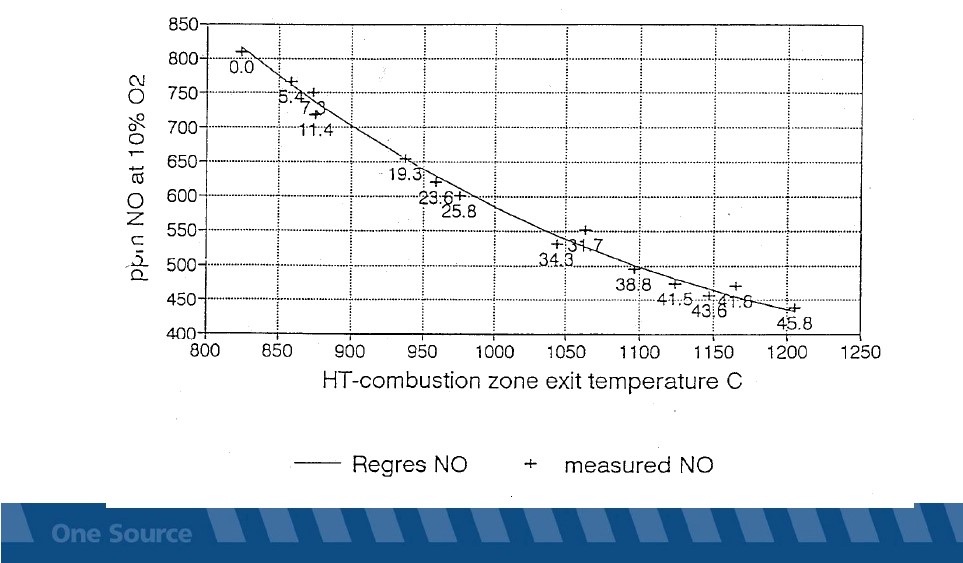
High Temperature Combustion in Calciner:
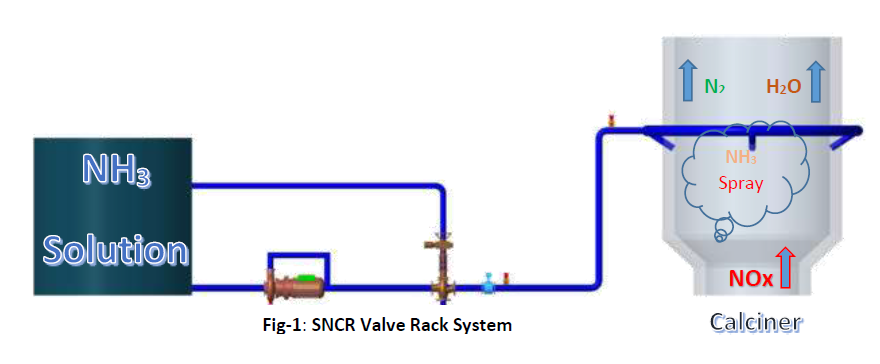
Selective Non-Catalytic Reduction System (SNCR):
It suffices to state that aqueous ammonia (NH3) is injected at a specific point in the process characterized by a suitable temperature window between 850 and 1050 °C depending on residence time, turbulence, oxygen content, and a number of other factors specific to the given gas stream.
In a selective non-catalytic reduction (SNCR) process of nitrogen oxides, reductants in an aqueous solution (ammonia water, urea) or in gaseous form (ammonia) are injected into hot flue gases. Following the overall post-combustion reactions for molecular nitrogen, water and carbon dioxide are formed. The optimum temperature range, where a noticeable NOx reduction is achieved >800 mg/Nm3, is between 850 and 1050 °C depending on the composition of the flue gas. Above this temperature range ammonia is oxidised to an increasing extent, i.e. nitrogen oxides are formed.
Urea NH2CONH2 + 2 NO + ½ O = 2 N2 + CO2 + 2 H2O
Or for
Ammonia 4 NH3 + 4 NO + O2 = 4 N2 + 6 H2O
At lower temperatures the reaction rate is slowed down, causing an ammonia slip which may result in the formation of ammonia salts in the further flue gas path and may lead to secondary problems. Therefore, the ammonia slip should be kept on a minimum.
Reactions (with Ammonia) when injecting NH3
if T = 950 ± 50°C
NH3 + OH - NH2 + H2O
NH2 + NO - N2 + H2O
NH3 + O2 - NO + H2O
if T < 900°C
SNCR System Cost per Ton of clinker
Base Capacity of Plant: 5000 tpd.
NOx reduction from 1150 mg/nm3 to 750 mg/nm3.
Ammonia % aqueous solution considered: 25 %
Expected Ammonia consumption: 0.92 m3/hr.
Cost of ammonia considered: USD 0.17 / Litre
Yearly Cost of NH3
0.92 x 1000 x 24x0.17x330 = USD 1.24 Million/ Year
Cost/Ton of Clinker = USD 0.733/T of Clinker
Ammonia consumption cost : USD 0.73/ton Clinker

Congratulations @aqueelansari! You received a personal award!
Click here to view your Board
Do not miss the last post from @steemitboard:
Congratulations @aqueelansari! You received a personal award!
You can view your badges on your Steem Board and compare to others on the Steem Ranking
Vote for @Steemitboard as a witness to get one more award and increased upvotes!