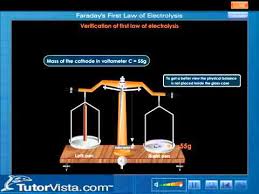
FARADAY FIRST LAW OF ELECTOLYTES
.jpg)
The mass of an element discharged during electrolysis is directly proportional to the magnitude of electric current..
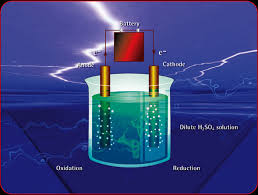
EXPLANATION
aCCORDING TO THE LAW
W × A × t
W = Z×A×t
Where
W = mass of the element deposited or li9berated
Z = electrochemical equivalent of the substance
A = amount of electric current in ampere
T = time in seconds