Alkali metals properties, [PART I].
INTRODUCTION.
The alkali metals belong to group I of the periodic table, since they possess a single valence electron. This characteristic makes all the elements of this group (including hydrogen), have an oxidation number +1 in all its compounds.
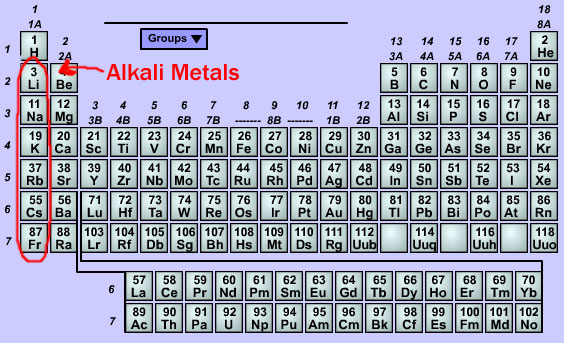
The alkaline metals are: Li, Na, K, Rb, Cs and Fr. In chemical reactions easily lose that electron, presenting very pronounced reducing properties. The radius of the atoms increase, as the atomic number of the element increases and in this same sense its reductive property grows.
The volatile salts of the alkali metals give to the colorless flame of the lighter colors characteristic of each elements: the compounds of sodium, yellow; those of lithium, carmine; of potassium, violet; rubidium, red-violet; and cesium, violet. this will be shown below.
METHODOLOGY.
-Identification of alkali metals by flame coloration:
LiCl, Na and K metallic were used for the identification of these metals by flame coloration, where a small portion of LiCl was taken with a spatula and placed in the flame of the lighter and the same procediment was made with Na and K metallic.
RESULTS AND DISCUSSION.
When placing LiCl in the flame, it took an intense red color, then with metallic Na the color of the flame was yellow and also very intense, finally, a piece of metallic K was placed on the flame which gave a violet coloring as shown in figures 2, 3 and 4 respectively. (I could´t find the image of LiCl I think I lost it).


The colors of the flame were the expected to identify each metal and this can be explein because at room temperature all the atoms of a sample of matter are in ground state. For example, under these conditions, the outermost electron of a sodium atom occupies the orbital 3s. the excitation of that electron to higher energy orbitals can be obtained using a flame, the life time of an excited atom is short and nevertheless its return to the ground state is accompanied by the emission of a radiation photon, the vertical lines of (figure 5) indicate some of the usual electronic transitions that follow the excitation of the sodium atoms, the wavelengths of the resulting radiation are also shown, the two lines at 5,890 Å and 5896 Å are the most intense and the responsible for the yellow color observed to expose sodium in a flame. The same happens for potassium and lithium with the difference that the photon emitted by each atom is more and less energetic than that of sodium respectively, that is why the color of the flame of the Li is red approaching the IR and that of the potassium is violet approaching the UV.

Finally, we did not use the other alkali metals (Rb - rubidium, Cs - cesium and Fr - Francium) because they were no avilable in the laboratory and they are more dangerous for being use.
I hope you to like this practice, I will be back with the second part.
REFERENCES.
- F.ALBERT Cotton, 1976, Química inorgánica avanzada, DF, México, Ed. limosa.437, 448p.
- Atkins, 2008, Quimica Inorganica, DF, Mexico, Cuarta Edición Mc Graw Hill. 258-272p
- Chang Rymond, 2007, Química, 9na edición, México, Ed. Mc Graw Hill, 336-337.
- Catherine E. Housecroft, Alan G. Sharpe, 2006, Química Inorgánica, Madrid España, Segunda Edición, Pearson Prentice Hall.
Yeah meet another chemist here!! Nice one!! upvoted and followed :DDD
I had recently made a post on alkali metals too ~
Feel free to drop by and have a look :D
btw, I am currently hosting a science picture contest with 30sp and ~20sbd prize. Feel free to see if you are interested :D
https://steemit.com/sciencepic/@mcw/christmas-science-picture-challenge-1-with-sbd-and-sp-prize-1-sbd-sp
Thanks @mcw, nice post yours too. metallic sodium as you show in your post is used to eliminate water when it is a problem in a solvent. I have the example of toluene when it is use for catalysis as reaction medium in polymers synthesis because the water affects drastically the behavior of the catalyst or even inactivete it.
Also I have videos where we study the reactivity of sodium on water and alcohol I will try to upload them on the other post.
Is that the use of organometallics as catalyst? Some of them are really water sensitive. Toluene sounds good to me as they are not as hygroscopic as THF, which is really a pain for me to use it as solvent.
Really look forward to your post !!! :))
Exactly for organometallic catalysis. Yes both of them are good but you have to considerate also the activity of the catalyst in different solvent before choose one, which in some cases could be very different. It is nice to meet other chemist.