When will the covid-19 vaccine become ineffective?
According to MSM and their expert panel after only a few months: a sharp decline from what they told you just three years ago (a full year of protection). That is why they told the Branch COVIDians not to rush out and get the KP.2 booster when it became available in late August. You’ll get sick this winter if you get the latest booster too soon they said.
Source: ABC News: The New COVID Vaccine is out. Why You Might not want to rush to get it.
It's important for those in high-risk groups to get vaccinated, but vaccine protection wanes after a few months. Those who run to get the new vaccine may be more likely to fall ill this winter when the next wave hits, said William Schaffner, an infectious disease professor at Vanderbilt University School of Medicine and a spokesperson for the National Foundation for Infectious Diseases.
And despite telling people to wait a couple months before getting the new booster they admitted the CDC program that pays for boosters for uninsured and under insured people who are unwilling or unable to pay $200 for it ran out of money by the end of August. So much for waiting to get boosted.
Of course, the vaxx isn’t what “wanes” in protection; your immune system is because supposedly the modRNA is cleared from your lymphatic system in a few days. The modRNA lipid particles don’t produce the cross reactive IgG antibody response to the spike protein; your immune system does after the modRNA LNPs transfect your cells and instruct it to produce spike proteins (and many off target frame shifted proteins) and presents them on the surface of your dentric cells. The phrase “vaccine protection wanes” is misleading and confuses people about what is actually occurring. What they don’t tell you is that after repeated exposure to the spike protein you eventually develop immune tolerance to it, a phenomenon that occurs after your antibody response becomes disproportionately focused on rare IgG4 antibody subclass switch. This rare antibody subclass typically only becomes more prevalent after repeated incremental exposure to non-pathological allergens. In fact, that is one of the goals of immuno-therapy. Allergen specific IgG4 antibodies are used as a biomarker in immuno-therapy because it inhibits other subclasses of antibodies from producing inflammatory responses that characterize allergies. However, it is also associated with autoimmune disorders, hematologic (blood) disorders, parasitic infections (e.g. malaria), and neoplasms (tumors).
Source: Frontiers Journal: The Clinical Significance of allergen specific IgG4 in allergic disease
IgG4 antibody prevalence also appears to mediate antibody dependent enhancement of disease and predicts COVID19 disease progression and mortality. IgG4/IgG1 ratio is an independent predictor of 30 day COVID-19 mortality; a blood concentration greater than 700 mg/dL and a ratio greater than 0.05 (1:20) is associated with a higher COVID-19 mortality after a 30 day follow up. It is also positively correlated with the serum concentration of Interleukin-6 cytokines that also independently predict higher COVID-19 mortality.
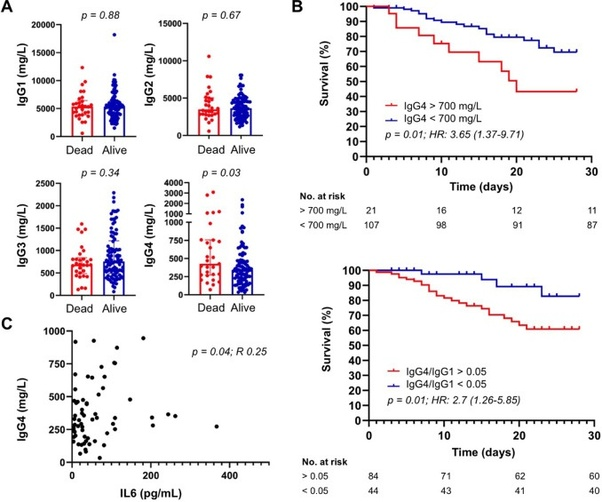
There are two possible mechanisms by which IgG4 antibody response can make disease prognosis worse:
Based on the available literature, IgG4 antibodies may contribute to COVID-19 progression via at least two possible mechanisms, yet to be verified. Because anti-spike IgG4 have shown poor in vitro neutralizing capacity compared to IgG1, IgG2, and IgG3 antibodies, a first possibility is that hosts with prominent IgG4 immune responses might be more permissive to SARS-CoV-2 infection . On the other hand, as neutralizing anti-IFNγ (interferon type II) auto-antibodies observed in adult patients with multiple opportunistic infections are predominantly of IgG4 subclass, it is tempting to speculate that anti-IFN antibodies associated with impaired anti-SARS-CoV-2 immunity and life-threatening COVID-19 pneumonia might also be IgG4 .
As I mentioned in a prior answer this permissiveness to SARS-COV-2 infection (and replication) is likely due its lack of an effector function that can activate complement immune pathways such as phagocytosis of infected cells and viral particles.
Higher levels of anti-receptor binding domain IgG4 antibodies are also positively associated with higher COVID-19 mortality at 8–14 and 15–21 days after symptom onset compared to patients who recover from severe COVID-19. Greater IgG4 proliferation is also found to occur with an increase of Interleukin-10 cytokines.
Surprisingly, we also noticed higher levels of RBD-specific IgG4 in sera from patients who died when compared to survivors in the second and third weeks. In our analysis, more that half serum samples of patients who progressed to death showed positivity to RBD-specific IgG4 antibodies, whereas most patients who recovered from COVID-19 were IgG4 negative to SARS-CoV-2 RBD in the same window of time.
This is not surprising because IgG4 antibodies have low binding affinity and do not form immune complexes or activate complement pathways. IgG4 levels have been found to significantly increase in proportion to other sub-classes 6 months after the second dose of the primary series and replace IgG1 as the dominate subclass of anti-receptor binding domain antibodies a few months after the second booster dose. Interleukin-10 cytokine concentrations also increase following the first and second booster doses.
As I pointed out in a prior answer, modRNA induced immune tolerance and occasional antibody dependent enhancement of disease only appears to occur for immunocompromised people who are vaxxed prior to infection which is the majority of people and only for recipients of modRNA transfections (i.e. Pfizer and Moderna) so this phenomenon is not evident for recipients of the NOVAVAX recombinant spike protein or recipients of the now withdrawn andenoviral vector shots (Astrazenca and Janssen).
A serological assessment of SARS-COV-2 specific antibody subclass concentrations for recipients of three doses of modRNA (Pfizer and Moderna) and a single recombinant spike protein dose (n = 20) and four doses of the recombinant spike protein (n = 18), assessed at least 6 months after the last dose found that while recipients of four doses of the recombinant spike protein (Novavax) had over 10x higher anti-spike IgG3 concentrations modRNA recipients had over 75x higher anti-spike IgG4 concentrations. IgG4 antibodies impair Fc-effector mediated immune responses of antibody dependent cellular phagocytosis, antibody dependent cellular cytotoxicity and antibody dependent complement deposition. To the contrary, the IgG3 subclass is responsible for 80% of viral neutralization.
Still in all, the modRNA transfections aren’t what are becoming less effective over a few months. The immune system of the recipient is becoming less effective by becoming more tolerant of the spike protein by switching the antibody response. As I pointed out in the same answer this is why observational research appears to find “waning” and negative vaxx effectiveness (i.e. increased disease risk) after 6 months.