Overview:
In the following article an overview of a paper is presented. It was a requirement for the presentation of a seminar in the Simón Bolívar University, Venezuela:
Synthesis, Characterization, Crystal Structures, and Catalytic C–C Coupling and Hydrosilylation Reactions of Palladium(II) Complexes Derived from CNC Pincer-Type N-Heterocyclic Carbenes
Rosenani A. Haque, Patrick O. Asekunowo, Srinivasa Budagumpi, Linjun Shao.
European Journal of Inorganic Chemistry, 2015, 19, 3169-3181.
Metal complexes derived from N-heterocyclic carbenes (NHC) have developed a growing interest in researchers during the past 20 years due to their wide range of applications. Particularly, it has been shown that Pd (II) complexes with this type of ligand have potential as catalysts in CC coupling reactions due to the nature of the metal center, which can be adjusted by modifying the oxidation state and strength of the NHC ligand.
Most studies have focused on the development of Pd (II)-NHC mononuclear complexes with the general formula [(NHC)2-PdCl2], where NHC is an compound derived from imidazole or benzimidazole. However, pincer-type tridentate ligands have recently been developed, and their popularity has increased due to the robustness and thermodynamic stability of the resulting complexes. The advantage of using pincers, and particularly CNC pincers, is the rapid creation of an electronically and coordinately unsaturated Pd (II) center, which is open to incoming substrate molecules.
The work of Haque et al. presents the synthesis of five salts of (benz) imidazolium precursors of pincer type CNC ligands, from the species shown in fig. 1 linked by pyridine units, by N-alkylation of the imidazole nucleus. Subsequently, binuclear complexes of Ag (I) were synthesized from the in situ deprotonation of the previous salts using Ag2O and through a transmetalation, the corresponding complexes of Pd (II) -NHC were obtained.
All the complexes were characterized by IR and NMR spectroscopy of 1H and 13C{1H}. By means of the first technique, the formation of the complexes of both Ag (I) and Pd (II) could be determined from the differences in the frequencies of the C = N and C-N bonds of (benz)imidazole and the C = N bond of pyridine, since the coordination of the free ligand to the metallic center produces a change in the vibrational modes of the molecule due to the rigidity of the interaction. On the other hand, NMR techniques allowed to evidence the formation of carbine, by means of the disappearance of the signal from one of the protons and the low field displacement of the carbon signal corresponding to the formation of the complexes. Three of the Pd (II) compounds could be characterized by X-ray diffraction, confirming the distortion of the square planar geometry around the metal center.
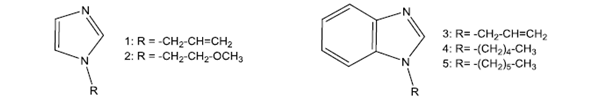
Fig. 1. Species derived from (benz)imidazole used in the synthesis of pincer ligands.
The catalytic potential of the Pd (II) complexes was demonstrated from its activity in the Suzuki-Miyaura and Heck-Mizoroki reactions in the aqueous phase, in which a cross-coupling between phenylboronic acid and n-butylacrylate was carried out with a series of substrates. For the first case, high yields were obtained (71-99%); while in the second, lower conversions were obtained (14-21%). Likewise, the catalytic potential of all the Pd (II) complexes in hydrosilation reactions was studied, obtaining moderate yields (37-57%).