Organic robots & the code of life
Not everybody knows about the existence of organic robots. Robots so small, so specific that they can distinguish between molecules that only differ in one atom and perform chemical reactions thousands of times faster than they should take place. Where can you find them? They are found by the billions just inside your body, keeping you alive. Of course, I am talking about proteins!
Figure 1. Design of a nanorobot. Not organic though, organic robots are cooler. Source: http://www.elconfidencial.com/tecnologia/2014-08-29/un-ejercito-de-nanorobots-para-localizar-y-destruir-tumores-desde-dentro_182422/
In this post I will go deeper into the mysteries of biology trying to explain how something as small as a protein, keeps you alive every day by performing tasks so amazing that defy human comprehension. But be careful, a small error in a protein can also cause the worst of illnesses, sometimes even death. I will also show you the code of life itself, literally. Buckle up friends and open your brains!
What are proteins made of?
Proteins are the smallest of robots, capable of performing a bewildering range of tasks - including synthesizing pretty much any other molecule your body needs, creating the main structures of your body (muscles, hair, tissue), acting as sensor and even relaying the electrical signals of your brain. – Damn that’s a lot of things!
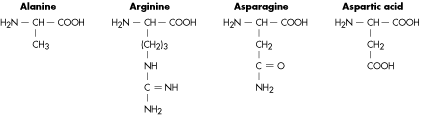
Figure 2. Structure of amino acids. As you can see the upper part is always the same, only the chain facing down changes. Source: http://www.tritechresearch.com/const.html.
The building blocks of proteins are amino acids, which are composed of the main atoms in organic chemistry: carbon, hydrogen, oxygen and nitrogen. -This is the same composition you mentioned for DNA in your old post!-. Yes, what changes is the molecule structure which you can see in Figure 2. A protein is made of sequence of amino acids which can go from hundreds to thousands.
The code of life
In a previous post I explained that DNA is organized in triplets and translated into amino acids, and thus, proteins. Well, this is the code of life lads:
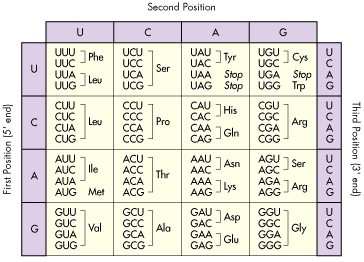
Figure 3. Code of life, for real. Triplet (or codon) to amino acid translation code. Source: http://www.tritechresearch.com/const.html
There are around 21 known amino acids, but 4 nitrogenous bases which are combined in triplets also called codons, hence 64 combinations. Thus, every amino acid is codified by several triplets or codons. In Figure 3, if you pay attention, you will see codons that are translated into Stop. These so-called stop-codons determine where the protein should end.
Enzymes – the most efficient robots
Once the sequence of amino acids is generated the proteins folds onto itself generating a 3-D structure that can interact with other molecules. Both the chemical composition and the 3-D structure determine to which molecules it will bind. There is no protein to rule them all and to the protein bind them, every protein is super specific.
Enzymes are proteins that catalyze reactions making them thousands of times faster. For example, making glucose react with oxygen to give you energy. Without enzymes, this reaction would take days instead of miliseconds. And you would be dead, of course. Now a bit of thermodynamics (you can skip this if you do not want to go so deep):
Enzymes lower the energy of activation of a reaction* -Sorry, whaaat?- Imagine you have a pile of 3 tons of dry wood. It will not burn by itself, does not matter how long you live it there. You have to use a flamethrower to start the fire. The energy you provide with the flamethrower is the activation energy. It starts a bigger reaction. The enzyme allows you to use a lighter instead of a flamethrower. In Figure 4 you can see how an enzyme lowers the energy required to start a reaction.
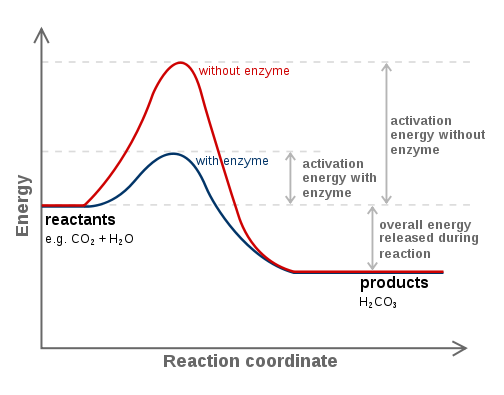
Figure 4. Effect on an enzyme on the activation energy of a reaction. Source: https://en.wikibooks.org/wiki/Structural_Biochemistry/Enzyme/Activation_energy
Extra chapter for the brave ones
One of the most amazing and first proteins whose function was one of the first to be understood is hemoglobin. This is the protein that transport oxygen from the lungs to the rest of your body. It has two states, relaxed (R) and contracted (T), which differ in the slightest torsion of the molecule (Figure 5).
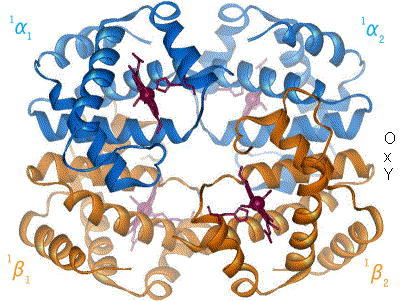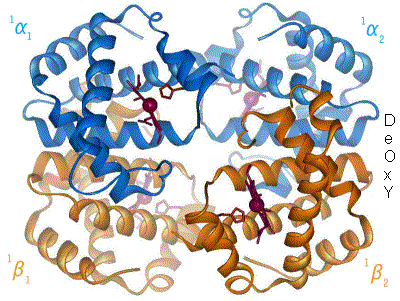
By en:User:BerserkerBen - Uploaded by Habj, CC BY-SA 3.0, Link
Figure 5. Change between T and R form of Hemoglobin. Source: https://en.wikibooks.org
The T form, has low oxygen affinity while the R form has high oxygen affinity. In the lungs, the concentration of oxygen is high, turning hemoglobin into the R form, which can bind more oxygen. When it arrives to the muscles for example, the concentration of oxygen is lower while the concentration of carbon dioxide is higher. This makes hemoglobin turn into T form, releasing oxygen and binding carbon dioxide. And this is how oxygen goes from you lungs into the rest of your body.
Hope you liked it! Do you have any questions? Do you want to learn more? Ask me and I will write about whatever you want to know. Follow me or check the rest of my articles to find out more about biotechnology
Other articles:
Bibliography
1 – Alberts, B., Johnson, A., Lewis, J., Raff, M., Roberts, K., & Walter, P. (1997). Molecular Biology of the Cell (Garland Science, New York, 2002).
2 – Berg, J. M., Tymoczko, J. L., & Stryer, L. (2002). Biochemistry. 5th. New York: WH Freeman, 38(894), 76.
Our body really do amazing things. We should really be looking into our lifestyle to ensure we are helping our nano-robots perform their tasks
@lemouth here you have a small intro on thermodynamics, as requested!
Thanks! That is once again a very clear post. I have however no specific question this time!
Very informative !
great article and very interesting topic! sometimes i wonder why people fly into space when there is a whole universe inside us..