The House of Halogens
Halogens

Colour Properties of Halogens
There a lot of things that we noticed every day but we do not understand. There are things that we take for granted without understanding how our lives would be affected without it. One such example is of halogens. Halogens are one of the most important discoveries known to man in the field of chemistry and plays an important role in the everyday lives of all of man. In this post I hope to elaborate your knowledge on these magnificent discoveries as well as to give you an insight into it individual adaptations to human life.
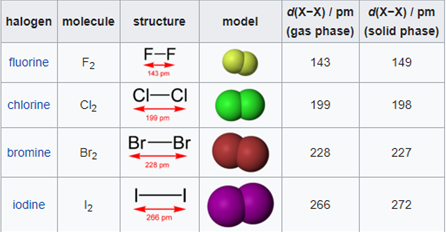
In the world of chemistry, a halogen is one of the most important aspects of interest. The group of halogens in the IUPAC group number 17 of the periodic table is uniquely the only group on the periodic table that, at standard conditions which are standard temperature and pressure (101325 Pa and 293.15 K), exist in the three main states of matter (solid, liquid and vapour).Halogens are vastly known for their salt formation properties, as the word halogen is derived from Greek descent which consists of the Greek words, hals and gennan, which directly translates to “salt” and “to form or generate” respectively. All halogens tend to form diatomic molecules and are all negatively charged ions in nature. In the group number 17 of the periodic table, the elements (periodically) are:
It may be it may be questioned as to why we included hydrogen in this list, as it was previously mentioned that halogens are in group number 17 only. To answer this question, we must understand the physical setup in which the periodic table was created, and this shall be explained in my next post regarding the periodic table. For now we should understand that the hydrogen element satisfies the properties of a halogen as, when chemically bonded to other elements, a certain form of salt is produced. It can also be noted that in group number 17, known as astatine is present. Although this element may be a salt former, it is generally not considered a true halogen because of its radioactivity. Most stable isotopes of astatine have half-lives of less than a minute. Due to this reason, it is not considered a halogen.
Halogens cannot be found in nature in the elemental form in nature. Instead, halogens are classified according to their halide ions. The reason for this is due to the fact that the salt formed from “halogens”, formed due to the chemical reaction between the ion of a halogen and an ion of another element. Since the produced salt can be found in nature, it is the salt that is studied, and in turn, the halide ion which will subsequently produce information regarding the halogen.
Fluorine ions are commonly found in many minerals, and are particularly studied in fluorite and cryolite. Chlorine ions can be commonly found in rock salt, the ocean and even in lakes that contain a high salt content. Bromide and iodide ions can both be found in low concentrations in the ocean’s as well as in brine wells in areas such as Louisiana, California and Michigan.
Since halogens are such an important building block in the chemistry of our world, it is of great interest for us all to have a basic understanding of each of these elements. Thus, a short description on each of the halogens in nature shall be explained:
Fluorine:
Fluorine is a colourless gas that is highly toxic and notably the most reactive element known to man. Due to its reactivity, as the ability to form compounds with elements that were thought to be inert gases (Xe, Kr, Rn). This phenomenon is quite astonishing as inert gases are unreactive with many elements. Florine is also a powerful oxidizing agent and is known to coax other elements into unusually high oxidation numbers in a redox reaction. Fluorine also produces many domestic products as it is used in manufacturing of polytetrafluoroethylene, or more commonly known as Teflon. Teflon is used in the production of everyday items from linings of pots as well as pans to industrial products such as gaskets that have the chemical properties of being inert to chemical reactions, thus forming part of a engineering process. Fluorine is also used to produce freons, which are used in the operation of refrigerators.
Chlorine:
Chlorine is a highly toxic gas with the macroscopic properties of being pale yellow-green. Chlorine is pronouncedly known for its strong oxidizing agent abilities as well as its use commercially as a disinfectant and bleaching agent. For example, chlorine is strong enough to oxidize the wood of trees to give the pulp of wood it’s yellow or brown colour and in doing so, not only does it complete its task of oxidation, but also bleaches the wood. It can also be noted, the chlorine to destroy bacteria in stagnant or moving waters as well as the air, thus, chlorine acts as a germicide. C can be used to make a large variety of products in almost every stream of industry, with its most important products being, carbon tetrachloride, chloroform, dichloroethylene as well as trichloroethylene.
Bromine:
Bromine originates from the Greek language, which consists of a stem word known as bromos, which translates to “stench”. It is then fitting to understand that bromine is an orange-reddish liquid that consists of an unpleasant and choking odor. Since bromine is a relatively heavy gas, it is often used to prepare flame retardant products, agents of fire extinguishers, used in the composition of gasoline products, sedatives as well as insecticides.
Iodine:
Iodine is a unique halogen as it is the first that we have encountered that has a solid form, with an almost metallic luster. Iodine, in its solid form, is a relatively volatile object and may form violet coloured gases when heated while sublimation occurs. Iodine compounds have been vastly traced to be used in many industries and quite frequently used in the engineering industry as iodine forms products such as catalysts, drugs as well as dyes. Compound containing iodine also play an important role, such as silver iodide, which are used in many photographic processes as well as used as an attempt to make rain by seeding clouds. Iodine also plays an important role in the human body as an iodine deficiency can cause swelling of the thyroid gland and lead to other severity.
A general trend be identified regarding the oxidation strengths as well as the reducing strengths of halogens:

An example of a Redox Reaction
When considering oxidizing strengths, there's a generalised decrease in the oxidising strength from fluorine to iodine:
Fluorine > Chlorine > Bromine > Iodine
When considering the reducing strengths of the corresponding halide, it can be noted that the trend above is mirrored, meaning, there is an increase in the reducing strength from iodide to fluoride:
Iodide > Bromide > Chloride > Fluoride
The halogens that we introduced in this post can often be referred to as reactive non-metals. An important point to consider regarding halogens is the reactivity and toxicity of each element. It can be noted once more, that the magnitude of reactivity as well as toxicity tends to decrease from fluorine to iodine. Fluorine can be noted to be a unique halogen with certain properties that are uncommon to the other halogens, such as:
- Fluorine is the most reactive of all halogens available.
- Fluorine-fluorine bonds are weaker than most other bonds such as chlorine-chlorine bonds.
- When fluorine reacts with hydrogen, to form hydrogen fluoride, the higher boiling point and strong intermolecular hydrogen bonds are noticeably higher and stronger than all other hydrogen halides.
- Fluorine can react with cold sodium hydroxide to produce oxygen difluoride.
- Fluorine can also react with chlorine or bromine to produce a halide as well as a hypohalite.
- When fluorine is chemically combined with silver, the resulting silver halide is soluble, whereas, when silver is mixed with any other halogen, forms an insoluble compound.
This brings us to the end of my posts on halogens. I hope that you now understand the importance and the individuality that these elements play as well as observe in our lives.
Images are linked to their sources in their description
The End
References:
[1]https://en.wikipedia.org/wiki/Halogen
[2]https://chem.libretexts.org › ... › Elements Organized by Block › p-Block Elements
[3]https://www.lenntech.com/periodic/elements/f.htm
[4]https://www.quora.com/What-are-some-examples-of-the-most-reactive-non-metals
[5]https://www.khanacademy.org/.../oxidation-reduction/redox-oxidation-reduction/.../o...
[6]https://chem.libretexts.org/.../Reactions_of_Main_Group_Elements_with_Halogens




Thanks for presenting me with the moment of nostalgia reading about this slice of chemistry. These are powerful compounds - the salts that contain them and the metallic part of the molecules also.
Thank you!
Chemistry is nostalgia on its own, it never seizes to amaze me about for simple matter such as these can provide such important and distinct roles in our lives. Thank you sir for taking time out to read my post, i really do appreciate it
Salt forming group.This post had shown to us fundamental knowledge in halogen. 👍 @akeelsingh
Thank you my fellow sir, it is of this fundamental components that our lives continue in leisure. Thank you for affording me your time fellow friend!