REACTIVE METALS: Solubility of Ionic Salts
Most ionic salts dissolve in polar solvents such as water. A solvent is a liquid that can dissolve other substances. The most common solvent is water. A solute is the substance that is dissolved the solvent. A solution is the name given to the mixture of the solute and the solvent. For example, a sodium chloride solution is simply a mixture of water (the solvent) and sodium chloride (the solute).
SOLUBILITY
The solubility of a substance is defined as the maximum concentration that can be obtained when the substance (the solute) is dissolved in a solvent. This concentration is usually measured either in grams of solute per 100 grams of solvent or in mol dm-3. Throughout this article, the chosen unit of solubility is mol dm-3.
Solubility changes with temperature, so it should always be quoted at a particular temperature, usually 298 K.
THE DISSOLVING PROCESS
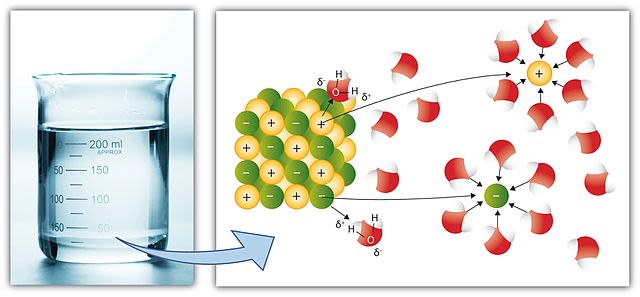
This section considers what happens when an ionic salt dissolves in water. In the regular arrangement of an ionic lattice, the cations are electrostatically attracted to the anions. When an ionic salt dissolves, these electrostatic forces of attraction are broken and the anions and cations are free to move throughout the solution. In a polar solvent, such as water, the ions are surrounded by water molecules.
Since the electronegativity of oxygen is much higher than that of hydrogen, the oxygen atom of a water molecule carries a small negative charge and so is weakly attracted to the cations. The hydrogen atoms of a water molecule carry a small positive charge and so they are weakly attracted to the anions. As a result, both cations and anions are surrounded by many water molecules. When sodium chloride dissolves, aqueous sodium ions and aqueous chloride ions are formed.
Overall, the dissolving process can be considered to be two processes: one is the breaking of the electrostatic attraction between ions in the ionic lattice; and the formation of aqueous ions with attractive forces being established between the water molecules and these ions.
ENERGY TRANSFERS DURING DISSOLVING
When an ionic compound dissolves in water, there is always an energy transfer. Normally, the energy transfer process is modest and therefore there is only a small change in the temperature of the water. Energy is required to break the electrostatic attraction between ions, and energy is released when the attractive forces between water molecules and the ions are established. The numerical value for these two energy transfers (one endothermic and the other exothermic) is often very similar so that the overall energy change when making a solution is small. Occasionally, a dramatic change occurs in water temperature, but this is nearly always because a chemical reaction is taking place during the formation of the solution.
The enthalpy change of solution, ΔHsoln is defined as the enthalpy change when one mole of solute is dissolved in a solvent and extra dilution causes no further change in enthalpy.
Entropy and dissolving
Entropy is described as a measure of the disorder of a system. When an ionic solid dissolves to make a solution, the system becomes more disordered (increases in entropy), because all the particles in the solution are free to move, whereas in the solid the particles were in fixed positions (lower entropy). Processes in which the entropy – the amount of disorder – increases are favoured over those in which the entropy does not increase. This means that all substances should dissolve in a solvent. But this is not the case because entropy changes in the surroundings must also be taken into account. The energy transfer processes offer a simpler explanation.
BORN-HABER CYCLES FOR DISSOLVING
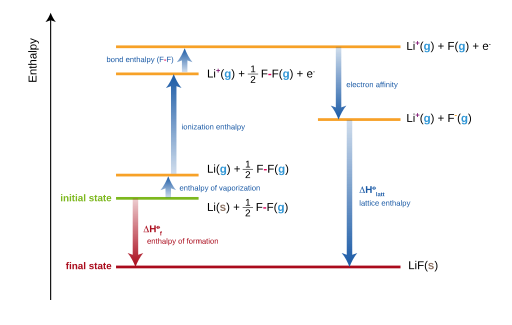
The enthalpy change of solution is equal to the sum of three different energy transfer processes. The first of these processes is the lattice enthalpy, the second is the enthalpy change of hydration of the cation and the third the enthalpy change of hydration of the anion. The enthalpy change of hydration ΔHhyd is the enthalpy change that occurs when 1 mole of gaseous ion is dissolved in excess water to make one mole of aqueous ion.
Although the enthalpy change of hydration is very much a theoretical energy change, it does give a clear indication of the strength of the interaction of the water molecules with the ions. The enthalpy change of hydration is always exothermic. Since it necessarily involves bond-making between water molecules and the ions. The lattice enthalpy contribution to the enthalpy change of solution is always endothermic, since it involves the breaking of the strong electrostatic interactions between the positive and the negative ions. The lattice enthalpy and the sum of two enthalpy changes of hydration have roughly the same magnitude (but are of opposite sign), so that the enthalpy change of solution is usually very small.
Enthalpy change of hydration and charge density
The magnitude of the enthalpy change of hydration depends on the ionic radius and the charge on the ion. The smaller the ionic radius and the higher the charge on the ion, the more exothermic the enthalpy change of hydration. This is because an ion with a small radius and a high charge has a high charge density, and therefore water molecules are strongly attracted to it.
Conversely, an ion with a large radius and a small charge has a low charge density, and therefore water molecules are weakly attracted to it. Within Group 2, the enthalpy change of hydration of M2+ becomes Iess exothermic as the atomic number increases:
SOLUBILITY TRENDS OF THE GROUP 2 SULPHATES
It is stated in my previous post that the solubility of the sulphates decreases as the atomic number of the Group 2 elements increases. This trend can be explained in terms of the energy transfer processes that take place during dissolving.
Taking SO42- as an instance, the enthalpy change of hydration of the SO42- (g) is common to alI Born Haber cycles for all of the Group 2 sulphates, so it cannot be responsible for the solubility trend. The lattice energy of the Group 2 sulphates, MSO4 (s), becomes less exothermic as the atomic number of M increases, but the change in lattice energy from MgSO4 to BaSO4 is quite small because of the large size of the sulphate ion. However, the difference in the enthalpy change of hydration of M2+ (g) from Mg2+ to Ba2+ is large because of the big difference in ionic radii between the very small ion Mg2+ and the large ion Ba2+. It is the enthalpy change of hydration of M2+(g) that determines the solubility trend. The more exothermic this process, the more likely MSO4(s) is to dissolve.
To sum up: the enthalpy change of solution of MSO4(s) becomes less exothermic as the atomic number increases, and hence the solubility decreases. For the same reason, all salts of Group 2 elements that have an anion with a large ionic radius show the same solubility trend as the sulphates.
SOLUBILITY TREND OF THE GROUP 2 HYDROXIDES
The solubility of hydroxides, M(OH)2(s), shows the opposite trend to that of the sulphates. Namely, as the atomic number increases, the solubility increases. This time, the key factor that determines solubility is the lattice enthalpy of the M(OH)2(s) rather than the enthalpy change of hydration. Since the hydroxide ion is small, there is a considerable difference between the very highly exothermic lattice enthalpy of magnesium hydroxide and the relatively small exothermic lattice enthalpy of barium hydroxide. A large exothermic lattice enthalpy makes a large endothermic contribution to the enthalpy change of solution and so reduces solubility. Other salts of Group 2 elements with small anions, such as the fluorides, display a solubility trend similar to that of the hydroxides.
SOLUBILITY PRODUCT
The term ‘insoluble’ is not very suitable, since no ionic substance is completely insoluble in water. The dissociation process takes place even with substances referred to as insoluble, such as barium sulphate and calcium carbonate. Sometimes, the term ‘sparingly soluble’ is used to express the fact that the solubility of a substance is low. The term ‘insoluble’ should be taken to mean that the solubility of a substance is extremely low.
When calcium carbonate is shaken up with distilled water and left to settle, the water contains aqueous calcium ions and aqueous carbonate ions in very small concentrations. A dynamic equilibrium is set up that is best represented by the following equation:
CaCO3(s) ⇌ Ca2+(aq) + CO32-(aq)
An expression can be written for the equilibrium constant for this process:
K = [Ca2+(aq)][CO32-]/[CaCO3(s)]
The concentration of a solid is a constant and therefore can be included in a modified equilibrium constant known as the solubility product, Ksp:
Ksp = [Ca2+(aq)][CO32-(aq)]
The solubility product is the product of the concentrations of the aqueous ions formed when an insoluble or sparingly soluble ionic substance dissolves in water. Each concentration is raised to the power shown in the dissociation equation. For example, the dissociation equation for barium chromate(VI) is:
BaCrO4(s) ⇌ Ba2+(aq) + CrO42-(aq)
Therefore: Ksp(BaCrO4) = [Ba2+(aq)][ CrO42-(aq)]
CALCULATING SOLUBILITY PRODUCTS
The solubility product of a salt can be calculated by substituting the appropriate concentrations into the expression for the solubility product. The solubility product has a unit that depends on the expression for the solubility product. In this case, it is concentration squared. The unit can be worked out by substituting the initial units roto the expression for the solubility product:
Ksp(CaCO3) = [Ca2+(aq)][CO32-(aq)]
= (mol dm-3)(mol dm-3)
= mol2 dm-6
EXAMPLE
What is the solubility product for calcium carbonate, given that the solubility of calcium carbonate at 298 K is 6.9 × 10-5 mol dm-3?
ANSWER: Assume that excess calcium carbonate is shaken with distilled water until equilibrium is attained. The solubility of calcium carbonate corresponds to the concentration of the aqueous calcium ions (or the aqueous carbonate ions) in the equilibrium solution:
CaCO3(s) ⇌ Ca2+(aq) + CO32-(aq)
At start (before shaking)/mol dm-3: 0:0
At equilibrium/mol dm-3: 6.9 × 10-5: 6.9 × 10-5
Ksp(CaCO3) = [Ca2+(aq)][CO32-(aq)]
= (6.9 × 10-5)(6.9 × 10-5)
= 4.8 × 10-9 mol2 dm-6
I will be pausing here for now. In the next post, I will discuss on the Barium meal and the Common-ion effect of reactive metals.
Thanks for reading.
REFERENCES
http://www.3rd1000.com/chem101/chem103w.htm
https://en.wikipedia.org/wiki/Solubility
https://www.sciencedaily.com/terms/solubility.htm
http://www.chem.ox.ac.uk/vrchemistry/energy/Page_25.htm
http://virtuallaboratory.colorado.edu/CLUE-Chemistry/chapters/chapter6txt-4.html
https://courses.lumenlearning.com/introchem/chapter/solutions-and-entropy-changes/
http://butane.chem.uiuc.edu/pshapley/GenChem1/L20/2.html
https://en.wikipedia.org/wiki/Born%E2%80%93Haber_cycle
https://revise.im/chemistry/eee/born-haber
https://secondaryscience4all.wordpress.com/2014/08/10/born-haber-cycles/4/
https://www.ibchem.com/IB16/04.53.htm
https://www.youtube.com/watch?v=-IhdcGgILIo
http://www.ocr.org.uk/Images/377890-enthalpy-changes-of-dissolving.doc
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1184297/
https://www.chemguide.co.uk/physical/energetics/solution.html
https://www.youtube.com/watch?v=aFTolR1jPtI
https://www.chemguide.co.uk/inorganic/group2/solubility.html
This post has been voted on by the SteemSTEM curation team and voting trail. It is elligible for support from @curie and @minnowbooster.
If you appreciate the work we are doing, then consider supporting our witness @stem.witness. Additional witness support to the curie witness would be appreciated as well.
For additional information please join us on the SteemSTEM discord and to get to know the rest of the community!
Thanks for having used the steemstem.io app and included @steemstem in the list of beneficiaries of this post. This granted you a stronger support from SteemSTEM.