How Does The International Space Station Gets Its Oxygen?
I have been watching the launch and subsequent capture of the Crew Re-Supply Mission 14 (CRS-14) for the past few days and wondered to myself:
Just where does the International Space Station (ISS) get its oxygen from?
Bottles of compressed gas would be a solution but the bottles have thick metal walls and are very heavy. Launch costs are high so I am sure the engineers of NASA and associated companies have figured out a much smarter solution than that.
As well bottles of highly pressurized gas are a potential safety hazard and not something you would really want on your space station.
So after doing some digging into the topic I learned how they do it.
Daily Oxygen Needs of Astronauts
But first it would be good to know just how much oxygen is needed each day.
The oxygen needs for a typical astronaut is 840 grams per day (ref). There are typically six astronauts on the ISS at any given time which means a daily oxygen supply of about 5 kg of O2 is needed per day and about 1800 kg of oxygen per year.
The launch cost of the Falcon 9 is about $2600 per kg. The costs for just the oxygen would be therefore about 5 million dollars.
If it was delivered solely via compressed gas bottles the additional weight be very significant.
Electrolysis of Water
Water is a molecule that is made up of 2 hydrogen atoms and 1 oxygen atom (H2O). Passing an electric current between two electrodes and through water will cause it to decompose into hydrogen gas and oxygen gas. The process is called electrolysis.
The equation and mass calculation for the chemistry-minded is:
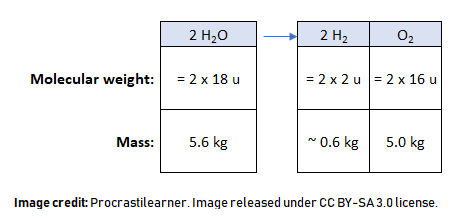
So for 5.6 kg of water the ISS can keep the entire astronaut crew supplied with oxygen for one day. The electricity is supplied by the solar panels which are powered by the Sun.
Electrolysis is therefore used because it is obviously much safer to transport non-pressurized, non-flammable water to the ISS than many bottles of heavy compressed gas.
The ISS uses at least two systems for making oxygen by electrolysis. The US-supplied and imaginatively named Oxygen Generator System (OGS) and the Russian made Elektron system (ref).

These systems either use water that was delivered straight to the ISS or it collects the water after the astronauts have consumed it and, ahem, excreted it (i.e. urine collection as well as the humid air that the astronauts breathe out).
Of course hydrogen gas is produced by the system and it is potentially flammable and under the right conditions it is also explosive. So this gas is vented out into space where it dissipates and becomes harmless. Goodbye problem.
Although you can never avoid the cost of the weight of the oxygen, with electrolysis you can at least avoid the cost of transporting all those heavy compressed gas bottles up into low Earth orbit.
Solid Fuel Oxygen Generator (SFOG)
There is an emergency backup system in case the main oxygen production systems fail and it is what is called an 'oxygen candle' or a 'chlorate candle'.
These are canisters that contain a mixture of powdered sodium chlorate (NaClO3) and powdered iron (Fe).
This SFOG mixture must be ignited and the iron powder burns at about 600°C. The heat is enough to cause the sodium chlorate to decompose into sodium chloride (salt) and oxygen gas (O2). Some of the produced oxygen must go into burning the iron powder and the remainder goes to keep the astronauts alive.
Again for the chemistry-minded the equation is:
2NaClO3 (solid) + Fe (solid) → 3 O2 (gas) + 2NaCl (solid) + FeO (solid)
(The equation only balances properly if you consider the Fe in the FeO 'steals' an O from the O2 gas generated for the astronauts.)

This system will give the astronauts time to fix the main systems, or failing that, get to the escape ship and leave for Earth.
Pressurized Tanks
Pressurized tanks are still used but in a limited manner. The OGS and Elektron systems produce only oxygen and a pure oxygen atmosphere is incredibly dangerous so this oxygen must be balanced out with nitrogen.
When the supply ships arrive at the ISS they pump compressed gases into the pressurized tanks at the airlock nodes. This system then monitors and mixes nitrogen into the ISS atmosphere at the right concentrations to keep the astronauts safe.
Plant Respiration
Animals breathe in oxygen and breathe out carbon dioxide. Plants 'breathe in' carbon dioxide and 'breathe out' oxygen.
Some tests of using plants on the ISS for respiration and a living gas cycle have been tried but to date plant growth problems has kept these attempts at the experimental level only.
Thank you for reading my post.
Post Sources
Reference 1: YouTube video
Reference 2:https://science.howstuffworks.com/oxygen-made-aboard-spacecraft1.htm
Reference 3: https://en.wikipedia.org/wiki/ISS_ECLSS
Reference 4: https://www.nasa.gov/pdf/570243main_OxygenGen_CHEM_ST.pdf
Thanks for an enlightening post. It's an interesting problem indeed. At least the source if solar energy is relatively constant which helps in calculating the power needed for the electrolysis.
That is interesting @procrastilearner. So compare to oxygen which would cost around 5 million dollar, how much they spend to transport water for producing oxygen up there?
I think that I kind of screwed up the point I was trying to make which was:
I think I will make an edit now.
Thx
Hahaha. Right.
Thanks for this informational post! It's a shame they have to throw away the hydrogen byproduct. In the future, we might find a way to use that as well somehow.
Hi, I found some acronyms/abbreviations in this post. This is how they expand:
Hi, I found some acronyms/abbreviations in this post. This is how they expand: