What is CRISPR-Cas9? – a historic introduction
With the advent of gene modification methods such as CRISPR-Cas9 new doors were opened for changing the genetic code of life. One should not forget that before the discovery of CRISPR several other techniques existed such as ZNFs (Zinc-finger nucleases) and TALENS (Transcription activator-like effector nucleases) that allowed gene editing. However, the clear advantage of CRISPR-Cas9 is its simplicity. This is because ZNFs and TALENS are restriction enzymes that can be engineered to bind any DNA sequence. When they are coupled to a endonuclease (a molecular scissor), this can make a double-stranded break in the DNA which can be used for modifying this sequence. However, the whole process was shown to be quite challenging and time-consuming.
CRISPR is the abbreviation for “Clustered Regularly Interspaced Palindromic Repeats”. In 1987, these palindromic repeats were identified in the genome of E. coli by Japanese researchers (Ishino et al., 1987).
A few years after, these repeats were discovered in various other bacteria but also archaea. Initially, researchers thought that they are part of DNA repair systems (Makarova et al., 2002). This suggestion was however shown to be wrong five years later.
In 2005, an essential discovery was made that eventually helped identifying the purpose of CRISPR. Researchers could show that the spacer sequences separating the palindromic repeats in the CRISPR loci had viral origins (Bolotin et al., 2005; Pourcel et al., 2005).
Furthermore, it had been demonstrated that the CRISPR loci are transcribed (Tang et al., 2002) and that cas genes (CRISPR associated) which are found upstream of CRISPR loci code for endonucleases (Haft et al., 2005).
Based on all of the above described aspects, CRISPR-Cas was suggested to be an adaptive immune system.
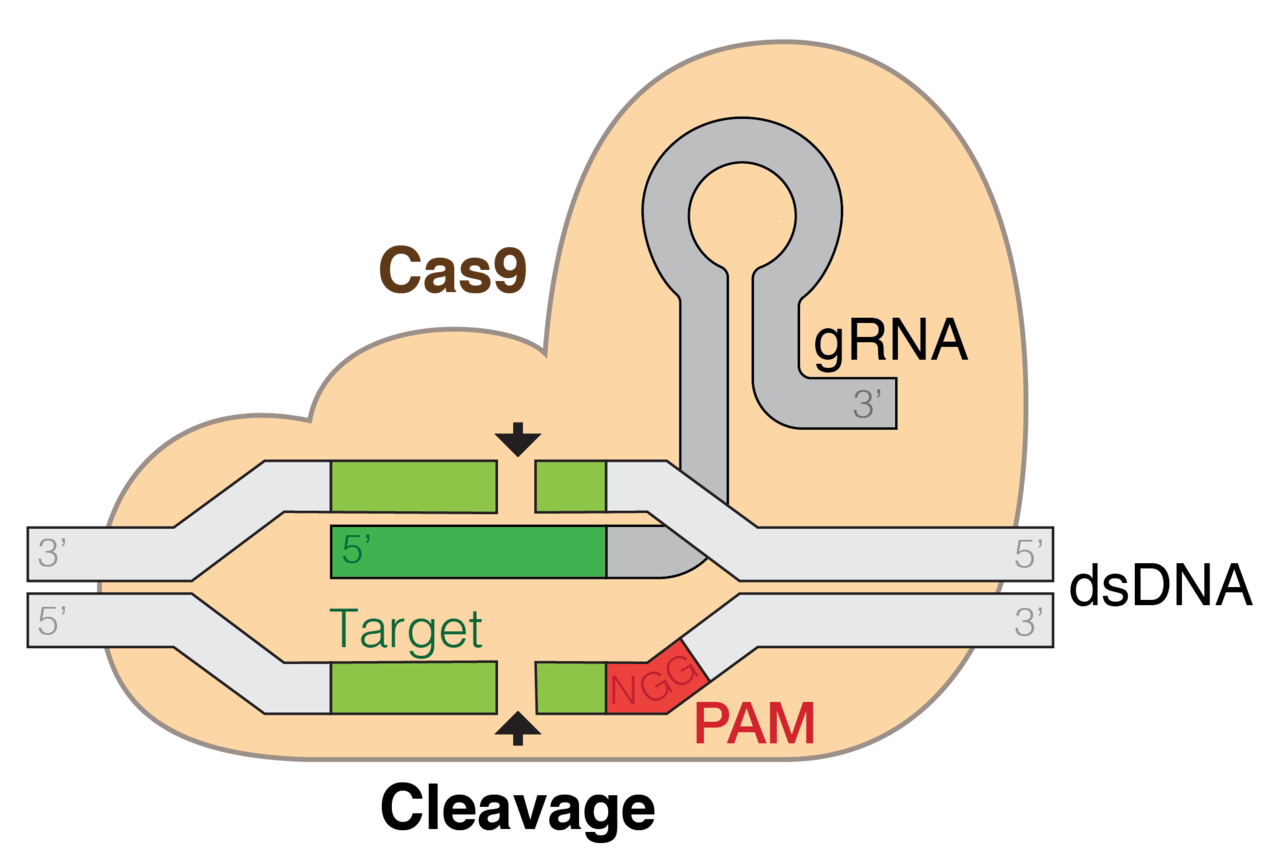
In 2007, a group of researchers could prove this hypothesis with experiments conducted in Streptococcus thermophilus (Barrangou et al., 2007). When the spacer sequence within the CRISPR loci are transcribed they are called CRISPR RNAs (crRNA). One year later, it was shown that these crRNAs can guide the Cas proteins to a complementary sequence on the viral DNA (Brouns et al., 2008). The binding of the Cas9 also depends on a protospacer adjacent motif (PAM), a short sequence that needs to be present next to the crRNA-targeted sequence.
However, it was not clear how the Cas proteins would interfere will the viral DNA or how they would stop the virus proliferation. Research on Cas had been conducted which now showed to be very useful. Cas9 which was one the initially identified Cas proteins was identified to contain two nuclease domains, HNH and RuvC-like (Makarova et al., 2006). Based on this, it was assumed that Cas9 interferes with the viral proliferation by introducing double-stranded breaks in the DNA (Garneau eta al., 2010).
In 2011, another important discovery was made. A second RNA was identified to be involved in the process of adaptive immunity in bacteria and archaea. This trans-activating crRNA (tracrRNA) which was found upstream of the CRISPR-Cas locus was proven to be necessary for the maturation of crRNA (Deltcheva et al., 2011.
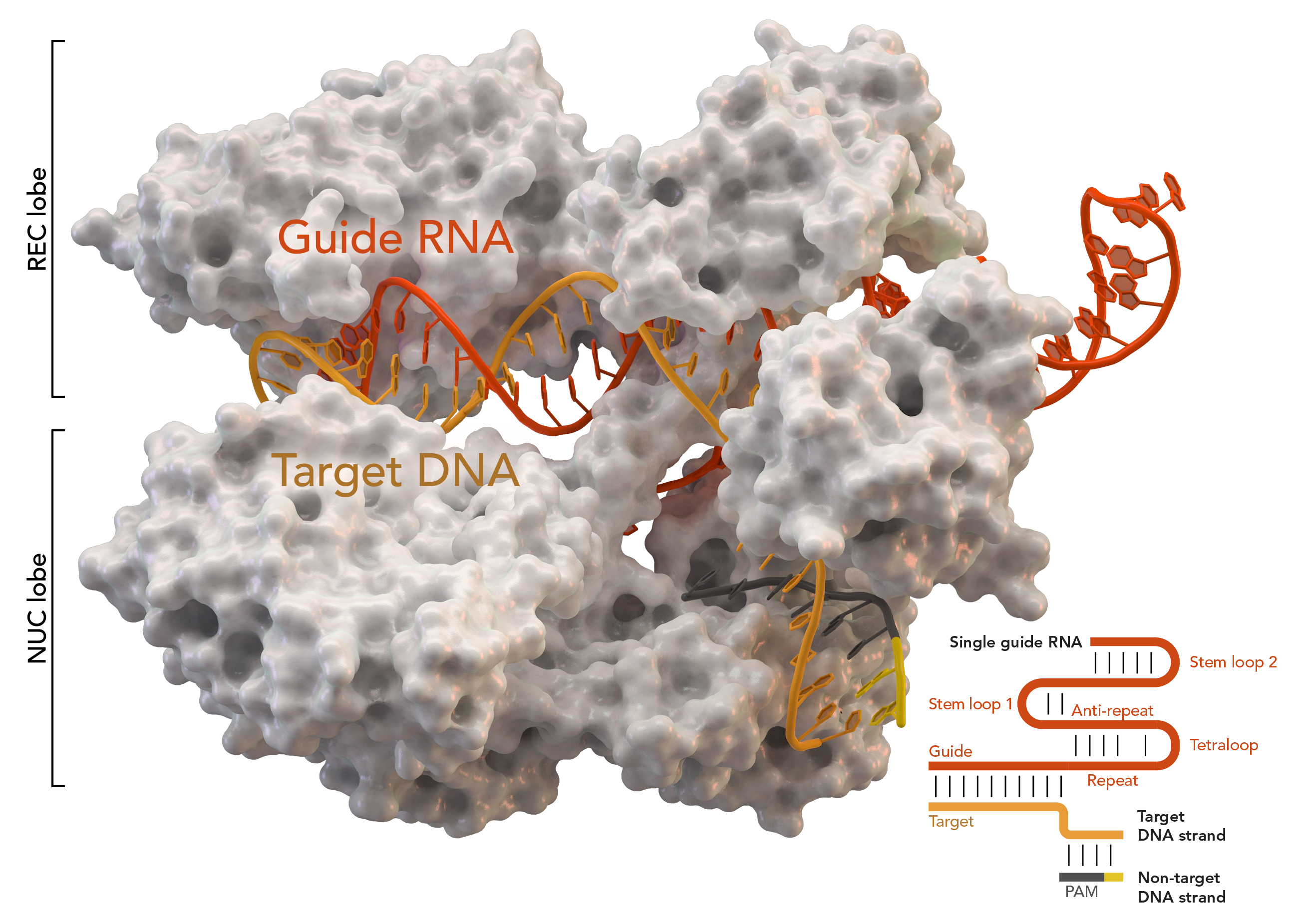
One year later, the lab group of Emmanuelle Charpentier and Jennifer Doudna could demonstrate that tracrRNA and crRNA form a duplex RNA that can guide the Cas9 enzyme to a specific target sequence (Jinek et al., 2012). Most importantly they showed that the RNA duplex was programmable, thereby opening a new door for genome editing.
The first reprogramming of human cells with CRISPR-Cas9 was conducted in 2013 (Jinek et al., 2013).
I hope you enjoyed this historic overview of the development of CRISPR-Cas9!
Being A SteemStem Member
You should also mention Jennifer Doudna as it was both her and Emmanuelle Charpentier's labs that contributed to the CRISPR "programmable nuclease" breakthrough in 2012. These two women are remarkable scientists and are very likely to win the Nobel Prize in Physiology or Medicine!
Cheers!
And Happy International Women's Day 2018
Thanks @rcebike for pointing that out. I just edited it.
Yes definitely. Their work is amazing and their truly deserve it!
For those who are curious about this amazing technology Eric Lander wrote a historical perspective on the "Hereos of CRISPR" and I encourage you to read it.