[English Version] Science for Everybody #9: Pyro- and Piezoelectricity

Die deutsche Version findet ihr hier.
Hey guys,
It’s been quite a while that I wrote an article in this series. However, I hope you appreciate another part, which will surely not be the last part. This time, I separated the English and German version, because otherwise, the post will be a little bit too long. The topic is a bit complicated but I hope that I can explain it to you.
Today’s topic
Today, we will talk about “pyro- and piezoelectricity”. Some of you might think now “What? What is this? How to pronounce it? And what has it to do with my everyday life?” Well, we look at it in the following article:
Meaning of the terms
I think, all of you has an association with electricity. Indeed, both terms have to do something with electric current. The prefixes pyro and piezo are of Greek origin and refer to fire (like in pyrotechnics) and to pressure, respectively. So, both terms are concerned with a property that links electricity to warmth and mechanical deformation, respectively. After a short explanation about symmetry, we will see, where this behavior comes from and what we can use it for.
Excursus: symmetry in crystals
This topic would surely be enough stuff for a whole article. Therefore, I will only give you some short information for a better understanding of the following sections.
So-called symmetry operations lead to the situation that an object has the same appearance before and after the operation. They are conducted on symmetry elements.
Let’s take a rotation as an example. It is made with a certain angle. The corresponding symmetry element is an axis of rotation. If you imagine a dye which has a point on each side (standing for the number 1) and you turn it by 360° it will look like before. Thus, it complies with our criterion. This is quite simple to understand and the rotation by 360° is a symmetry operation that is possible with every object.
If we fix the axis of rotation exactly through the 1 of our dye, other operations become possible: the rotation by 360/2 = 180° leads again to an image, that can’t be distinguished from the original (assuming that there are no defects in the dye). The object is in the original state after a twofold rotation. It is important that even the half rotation leads to an externally identical state. For this reason, this kind of rotation is not possible with any object. If it is possible, we call it a twofold rotation axis.
The last possibility to turn the dye is a rotation by 360/4 = 90°. The situation is analogously the same as for the twofold rotation axis: we have an externally identical object after each quarter turn and after four turns we find the original state. This fourfold rotation axis therefore contains always a twofold and onefold rotation axis.
In this position of the dye, there are no other turns possible that match our criterion. For example, a rotation by 360/3 = 120° (a threefold rotation axis) would lead to an easily visible state.
Besides these rotation axes, there are for example mirror planes that “copy” the right half of an object onto the left one and vice versa (or front to the back or top to down). Additionally, there are combined symmetry elements, like screw axes or rotoinversion axes. But these are not important for us at the moment.
Appearance of the properties
Both properties occur especially in crystals of certain minerals/salts that have a special structure (more precise: a special symmetry). Thus meaning, that they must not have certain mirror planes, for example. Because the structure leads to specific properties, we talk about structure-property-relationship.
In a former article, I explained that salts are built of anions and cations, meaning positively and negatively charged particles. Their arrangement is important for the here-discussed properties (another possibility to cause these are polar molecules, which are explained here, for example).
I will try to show you both properties by means of the compound zinc sulfide (ZnS). There are two kinds of zinc sulfide, although they consist of the same number of zinc and sulfur atoms (more precise: ions). These variants (so-called modifications) differ in how the atoms are assembled. We differentiate between the naturally occuring minerals Sphalerite (also known as Zincblende) and Wurtzite. Sphalerite is the cubic variant (its symmetry resembles a dye) and Wurtzite is hexagonal (its symmetry resembles a sixfold rotation).
Wurtzite and pyroelectricity
If we look at the so-called unit cell (this is the smallest building block of an ionic compound, which gives the whole structure if it is stacked side by side and on top of each other) of Wurtzite, we see a sequence of layers. These have an alternating longer and shorter distance. From a side view, we find layers like S-Zn – S-Zn – S-Zn and so on.
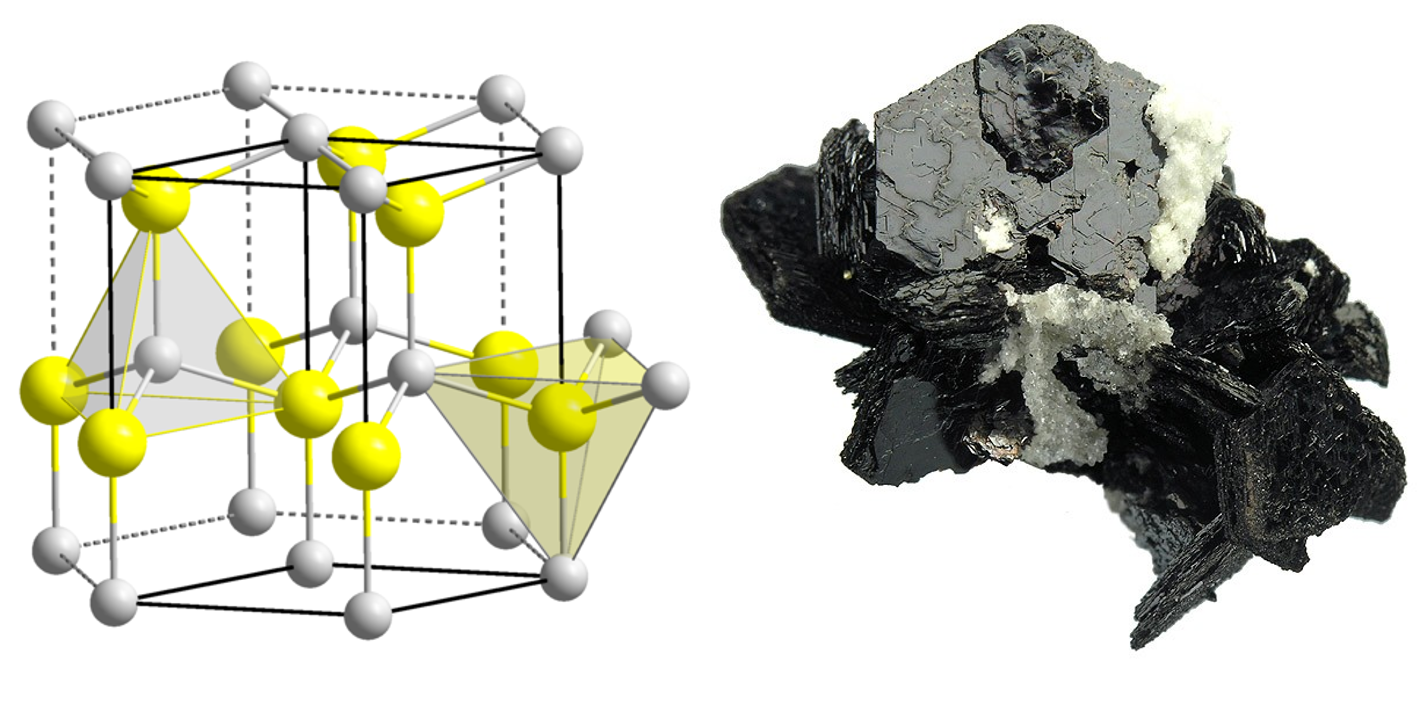
Left: picture of the unit cell (sulfur yellow, zinc grey), right: picture of the mineral.
Source: structure, mineral
This layer-like build-up, which can macroscopical (at visibly big crystals) be seen (see picture), causes two properties:
- The material tends to exfoliation (like e.g. schist).
- Wurtzite is pyroelectric.
How does it come to pyroelectricity and what exactly does it mean? In our compound, sulfur is existent as anion S2- and zinc as cation Zn2+. This causes that each of the ionic layers has an positively and negatively charged side. The whole mineral is electrical neutral because there are as many layers of each type. The above-mentioned layers can therefore also be written as +- +- +- and so on. We can see from this, that the whole material has a positive and negative side. Therefore, the mineral behaves like a big electrical dipole which can be contacted and measured.
If the temperature changes, the whole mineral expands or contracts, respectively. This is because of the stronger or weaker movement of the ions (see also here). Subsequently, the strength of the dipole changes and so does the measuring signal. This effect is called pyroelectricity.
Sphalerite and pyroelectricity
The unit cell of Sphalerite has a special atom-arrangement, too. I hope, you can see it a little bit on the picture. Let’s imagine a dye which is built from eight smaller dyes. You can see, that on every layer only two of the smaller dyes contain a sulfur ion and the other two are empty. Additionally, the filled dyes have a staggered arrangement.

Left: picture of the unit cell (sulfur yellow, zinc grey), right: picture of the mineral.
Source: structure, mineral
For example, in the upper layer, the filled dyes are in front left and back right position and in the lower layer, they are in back left and front right position (you may also look at the layers left-right or front-back and find the staggered arrangement in every case). Contrary, the zinc ions are equally distributed in the dye. Thus meaning, they are on all edges and in the middle of each surface (like a dye with six times number 1).
Due to this structure, the negative charge of the sulfur is distributed only in four of the eight edges. The dipole vectors, thus meaning their direction, show in these four edges, too. However, they neutralize each other by their symmetry (for those who want to know it more precise: via a rotoinversion or rotoreflection). To ease it: from every possible viewing direction, there are as many dipoles in as against the direction.
If we exert pressure on the crystal, the unit cell will be deformed in a way that, for example, one side is shorter than the others. Consequently, the fourfold rotation axis will be destroyed, and a twofold axis remains. Simultaneously, the dipoles are no longer equally distributed and equalize each other in every direction. Therefore, a positively and a negatively charged side of the crystal is build again, which can be measured. This phenomenon is called piezoelectricity. It is also possible to apply voltage to the crystal which then induces a deformation of the material.
Pyro- and piezoelectricity in everyday life
Both two phenomena may appear exotic but in fact, they can be found often in everyday life. Pyroelectrical materials connect warmth with electrical voltage and therefore, they can generally be used as sensor for temperature. Additionally, they may be applied as motion sensors that base on the measurement of warmth.
More often, piezoelectrical components can be found. These are for example used as igniter in lighters or as pick-up in e-guitar or -bass. The oscillating crystals in watches are based on piezoelectricity, too. The reverse effect, deformation by applying of a voltage may be used as actuator in highly precise devices. Some speaker (like in headphones) work with the piezoelectrical effect, too.
Terminating for now
I hope that I was able to explain you the topic in principal. For further information, I link you the relating Wikipedia articles. Please feel free to use the comment for questions and proposals.
See you next time!
Further information
Symmetry
Pyroelectricity
Piezoelectricity
Oscillating crystal
Piezoelectric speaker
Nice post..have a nice day brother..